Knowledge Base Improvements
Table of Contents
Improved Knowledge Base
The Castor Knowledge base is your platform for browsing articles, release notes, connecting with the support team via chat and much more. The platform has gone through an update and we are excited to share the improvements!
Sections Overview
The knowledge base is divided into several sections:
- EDC/CDMS
- eConsent
- SMS
- Helpdesk
- Status page
The EDC/CDMS, eConsent and SMS sections contain articles, FAQs, guides and compliance documentation related to each platform. The Helpdesk section contains some general information and latest news. The Status section links to the status page.
Article Quick View
Once you have located the relevant section, you can click on the article of interest and it will open in the right panel allowing you to preview the content:
To view the article fully, you can always click on the ‘Expand’ button:
Feedback form
Each article contains feedback options through which you can add a quick reaction or provide additional comments. Your feedback helps us to improve our knowledge base!
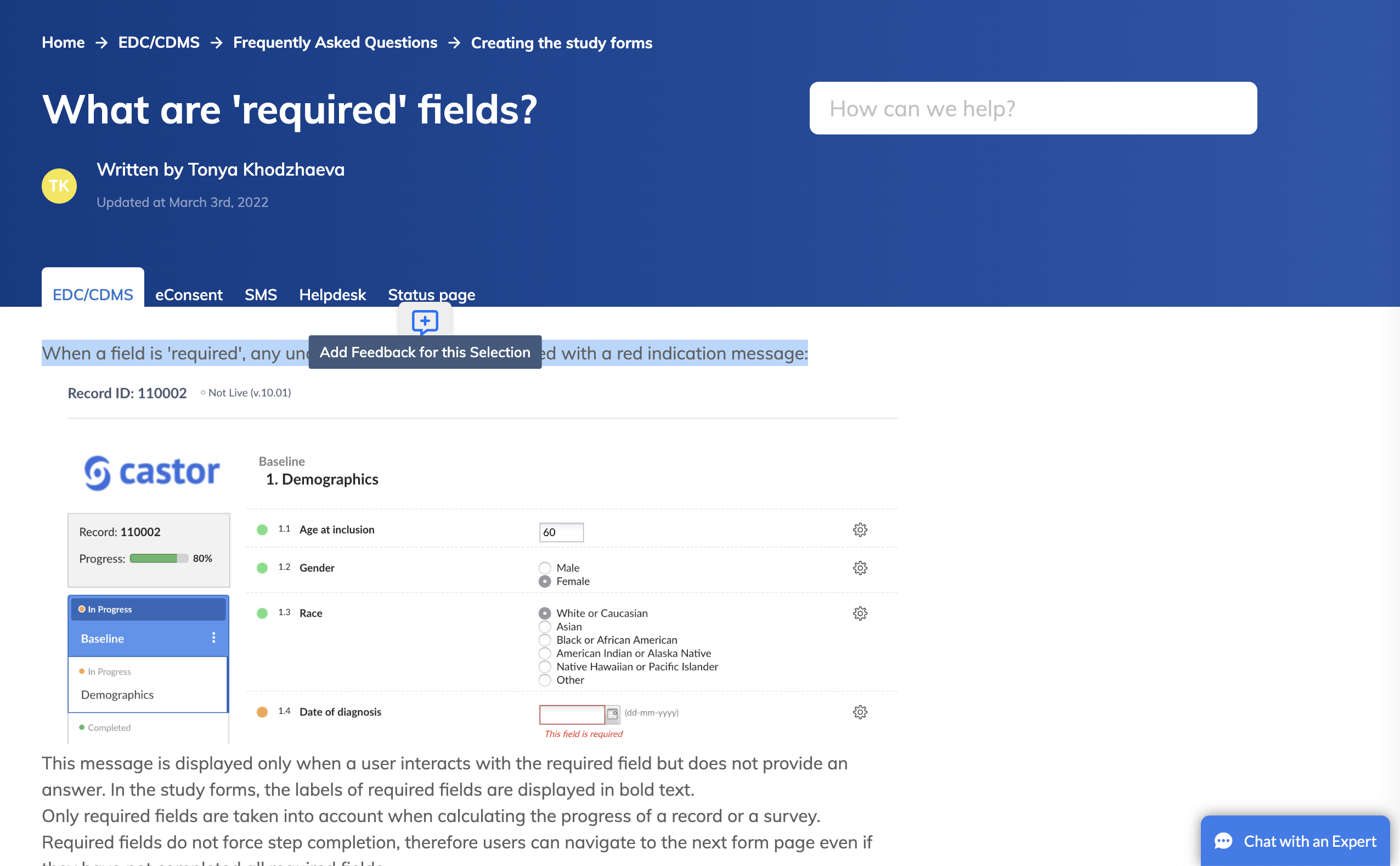
It is also possible to submit feedback about a particular section in the article. Just select the sentence or a paragraph and click on the comment icon to submit your comments: