Register and log in when invited to a study in CDMS
Table of Contents
A study admin can invite you to participate in the study by sending an invitation.
You will receive an invitation by email for the study for which you need to do data entry.
If you do not already have an existing account on the study's server then you will be required to create a new account. Additionally, you will also need to verify your email by clicking ‘Activate your account’ in the email that is sent during the registration process.
If you already have an existing account for the server the Study is on, login using the existing credentials for that server to access the Study overview page.
*Please note that when a study is Not Live, at least one management right is needed to be able to access a study, otherwise an error message will appear “Could not open selected study”.
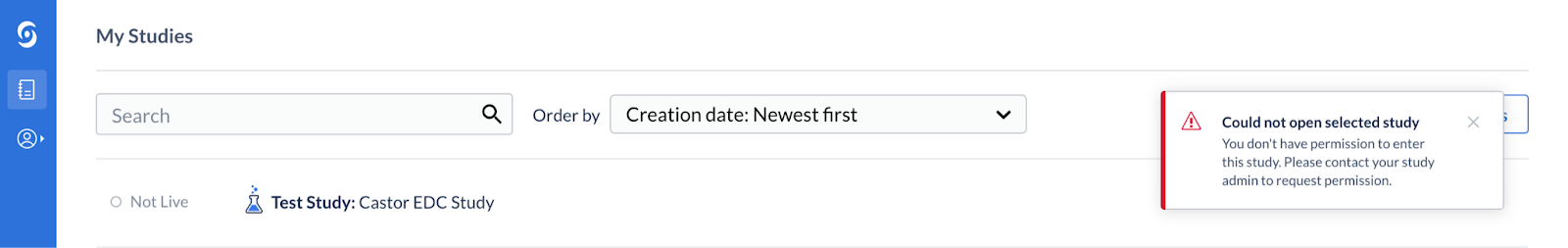
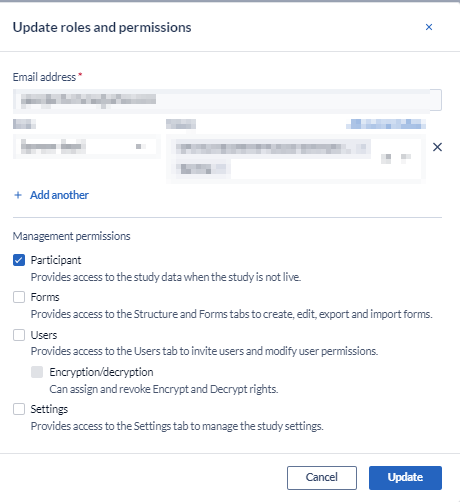
To correct this the Study Admin needs to change the Management Rights. See here for how to Define user rights.
Troubleshooting
If a study team member has been invited to a study, but has never received an invitation email, it is advised to consider the following scenarios:
- The email was sent to the recipient's spam folder. If an invitation email is found in the spam or junk folder, the recipient should move the email out of the spam folder and into their main inbox. In some cases, unmarking or moving this email to the primary inbox will indicate to the mail client that a study team member would like to receive these emails in the future.
- The email is filtered by the security filter of the recipient's server and is in the quarantine area before it could be released into the recipient's inbox. Some institutions have very strict emailing policies, adding the address from which the invitation emails are sent (no-reply@castoredc.com) as a safe email address should help resolve this. In some cases it is advisable to ask the recipient to register directly using one of the links below depending on the server where the data is stored. Once the study team member logs in, they will be able to see the study in the 'My Studies' overview. The list of registration pages based on the server:
- EU Server: https://data.castoredc.com/register
- UK Server: https://uk.castoredc.com/register
- US Server: https://us.castoredc.com/register
- AU Server: https://au.castoredc.com/register
- An incorrect email address was entered. Please make sure that the email address is correct.
- If after attempting the troubleshooting steps, the recipient is still not able to activate their account and access the study, please send a request to support@castoredc.com with the study ID (can be found in the Settings tab) and user email.