WHO Breakthrough cases Registry
Table of Contents
Step-by-step guide
As many COVID cases are not admitted to hospital, and to further address these uncertainties, WHO invites: Member States, health facilities, research networks and individuals to contribute anonymized individual data on cases of COVID-19 to this WHO Global Platform. Please note, if you already registered on the Castor EDC platform, you will still require new account for this WHO Registry.
Using this novel platform, WHO is conducting enhanced surveillance of cases of COVID-19 infection in vaccinated individuals globally, to confirm infection, identify risk factors and outcomes, and monitor their characteristics and to compare these cases to those in unvaccinated individuals.
Below you will find step-by-step instructions on how to submit your case.
For Healthcare Providers
Watch the video tutorial below:
Registering your interest and creating an account
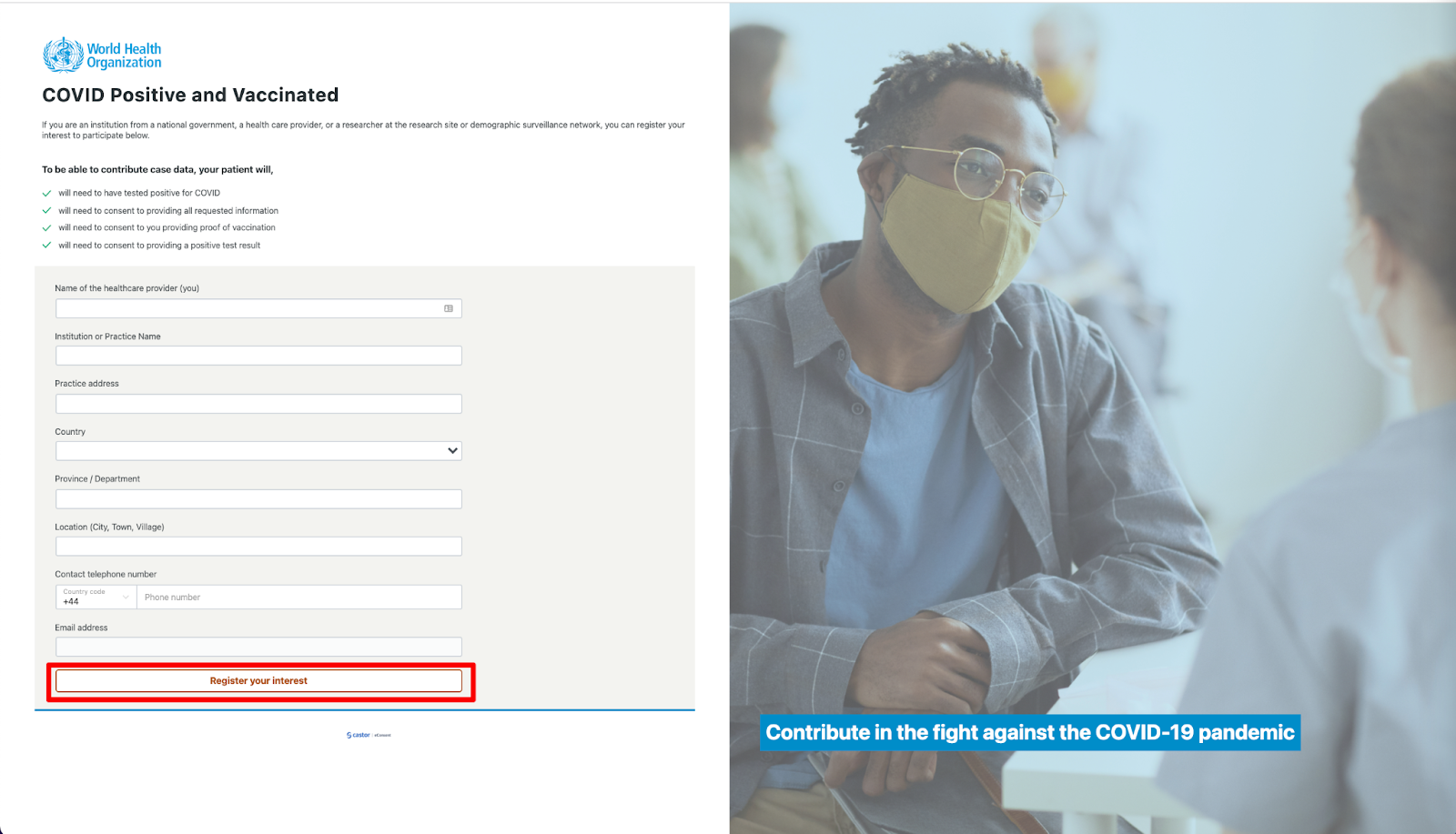
- If you are an institution from a national government, a health care provider, or a researcher at the research site or demographic surveillance network, you can register your interest to participate by clicking on the ‘Healthcare Provider’ button.
.png)
- You will then be redirected to the registration form where you need to add the following information: Name of the healthcare provider (you), Institution or Practice Name, Practice address, Country, Province/Department, Location (City, Town, Village), and Contact telephone number
- Click on the button ‘Register your interest’ to submit your details
- After you have registered, you will receive an email with the activation link.
- Click on the link in the email you received. You will be redirected to a form for activating your account on the platform.
- Add your First name, Last name and Password. Your password should contain at least 8 characters and have 1 uppercase, 1 lowercase and 1 numeric character.
.png)
Submitting a case
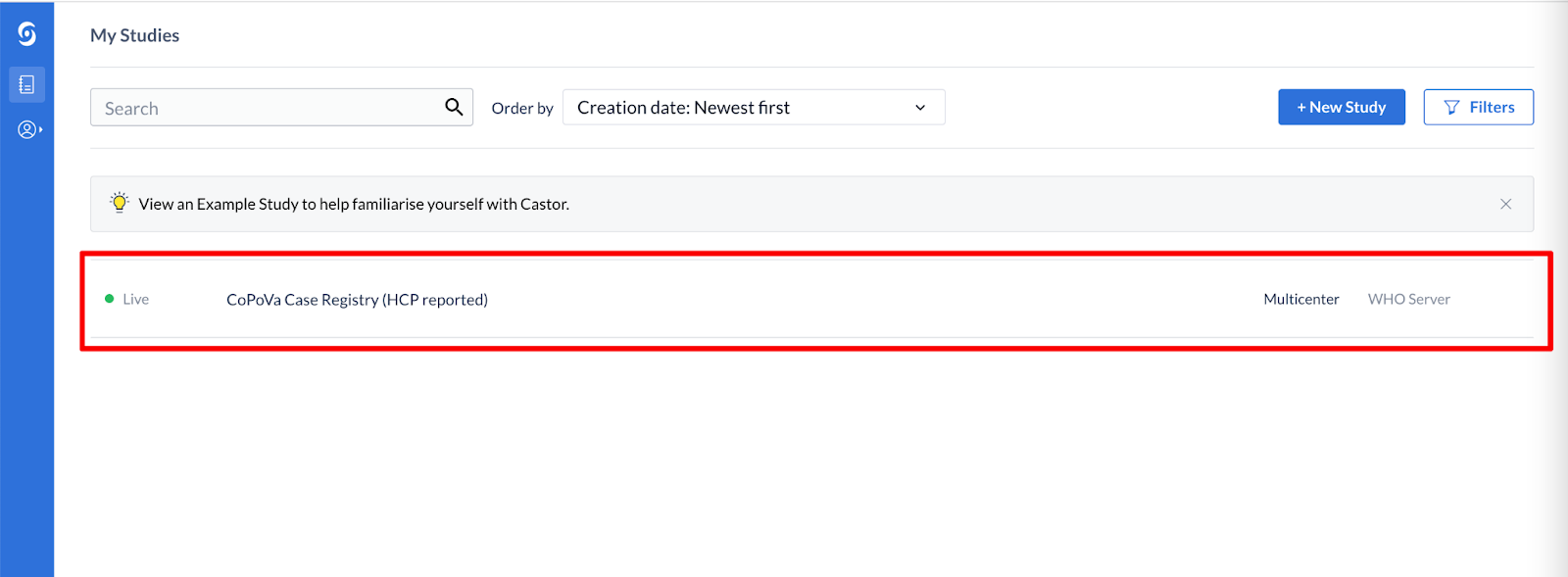
- After creating your account, you will be redirected to the My Studies Overview page.
- Click on the CoPoVa Case Registry (HCP reported)
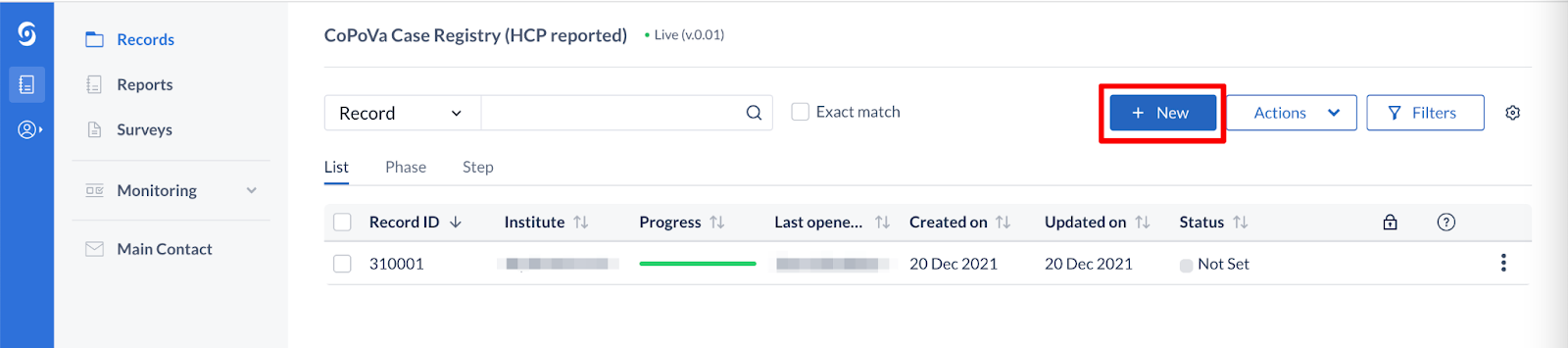
- Click on the ‘+New’ button to submit a new case
- Once you click on 'Create', your participant will be created and will appear in the 'Participants' tab overview. The ‘Email’ field is optional and can be skipped.
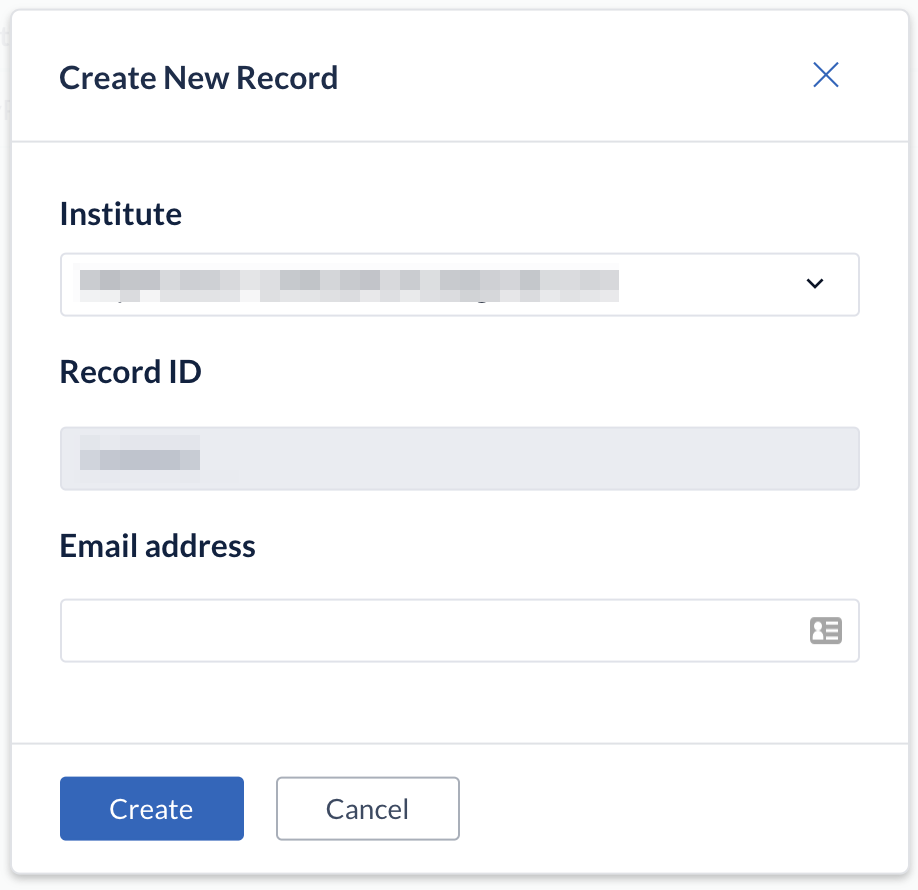
- When you open a participant, the data entry view is displayed. This is the main data entry view. It consists of the following elements:
- participant number and participant completeness
- Here you can navigate through the eCRF and see the individual progress form. Please complete the forms: Consent, Patient information, Vaccination status, Previous COVID episode, Current COVID episode.
- The fields added to the form - please complete these fields. The data will be saved immediately after entry.
- Field options, here you can perform various actions that apply to only the field on this row, e.g. ‘Clear data’ or 'Set field as missing'.
- You can use the Next/Previous buttons to navigate through the forms of the participant. Once you have reached the final form, the ‘Next’ button will be greyed out.
- The ‘Back to Participants’ button returns you to the participant overview list.
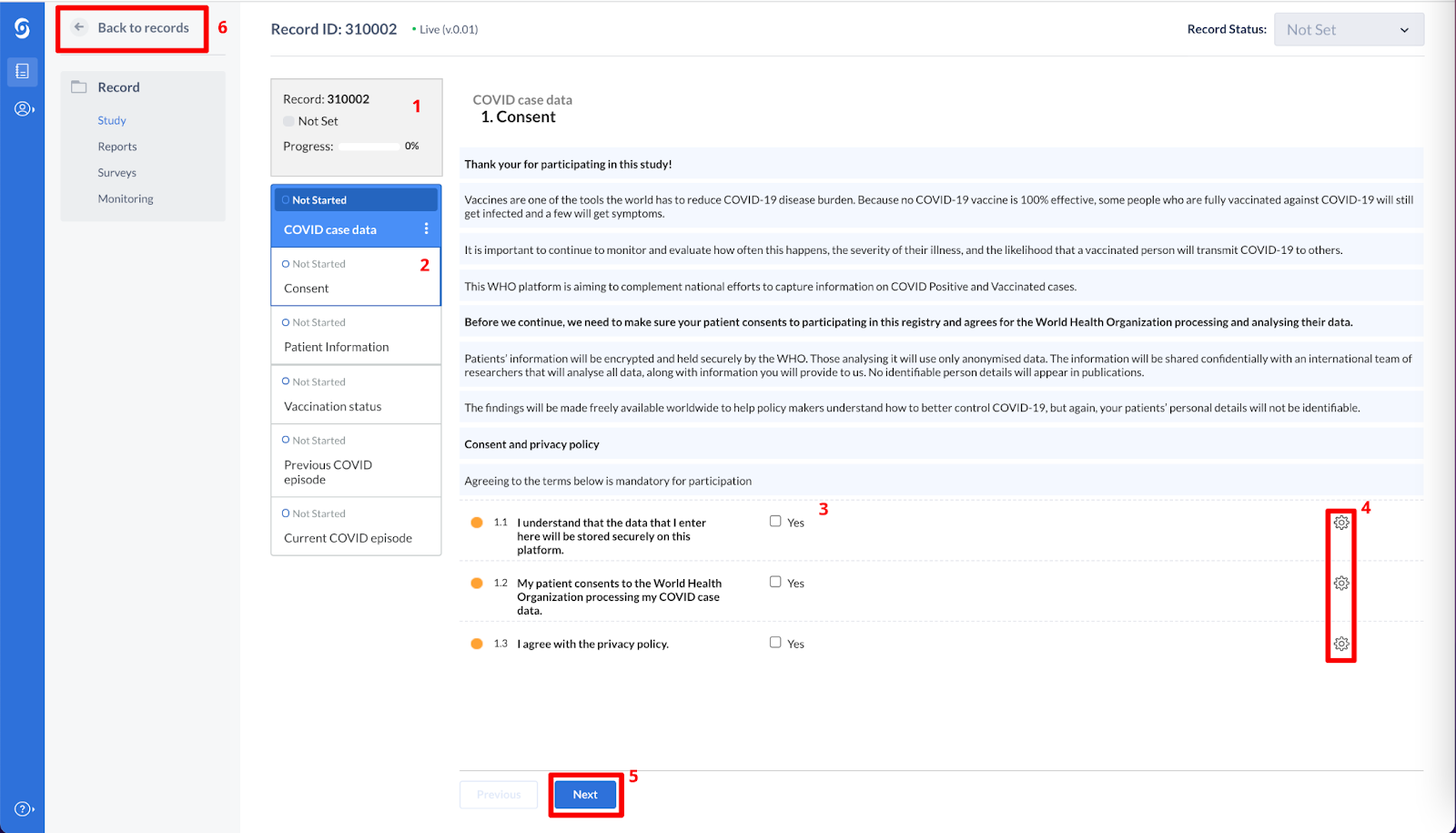
- Once you have completed all the forms for this case, you can click on the ‘Back to Participants’ button to return to the list of Participants.
For Individuals
If you are an individual who is vaccinated and recently tested positive for COVID, you can contribute your case data by enrolling in this project
Watch the video tutorial on how you can register your case:
Step-by-step instructions
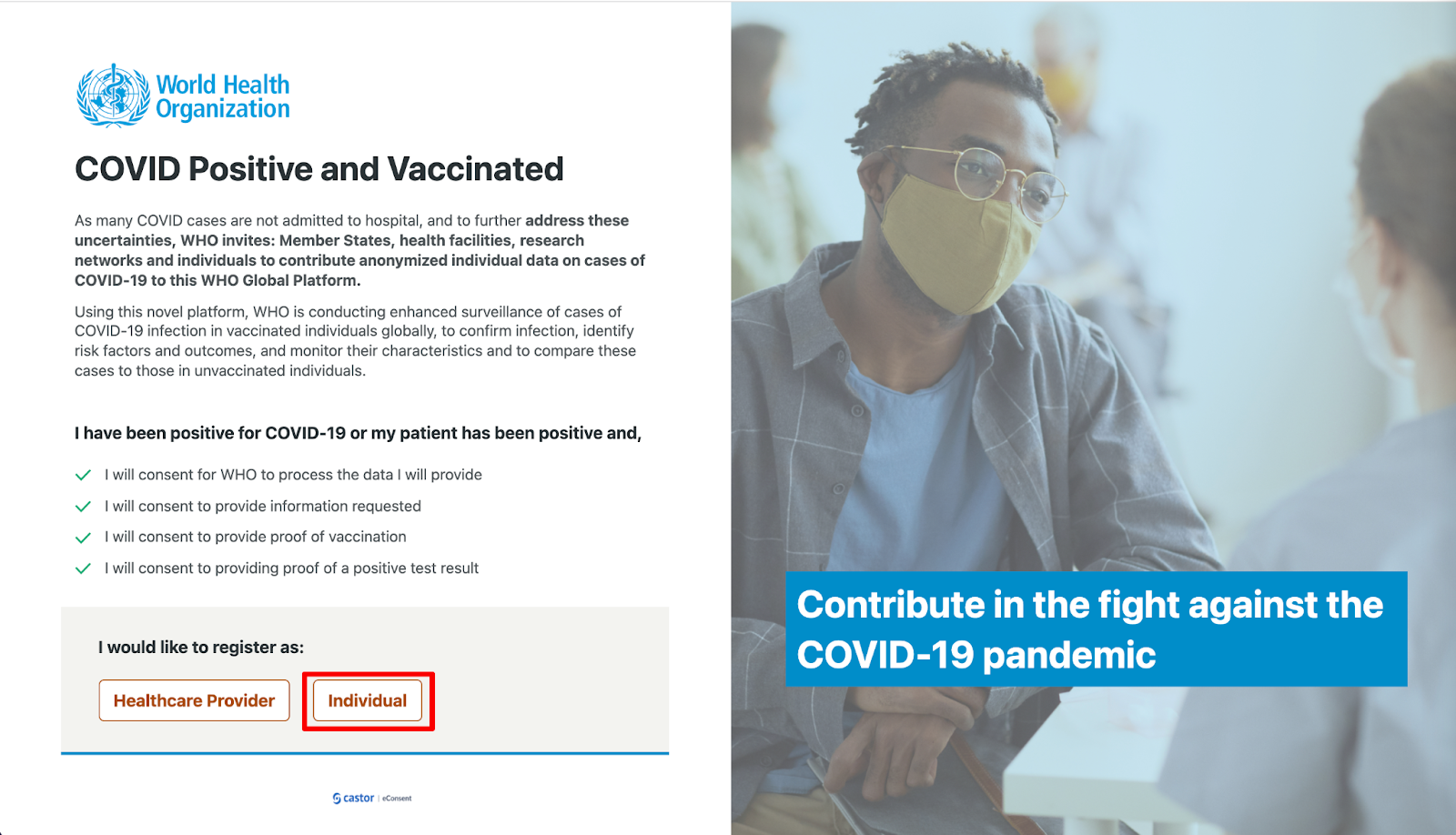
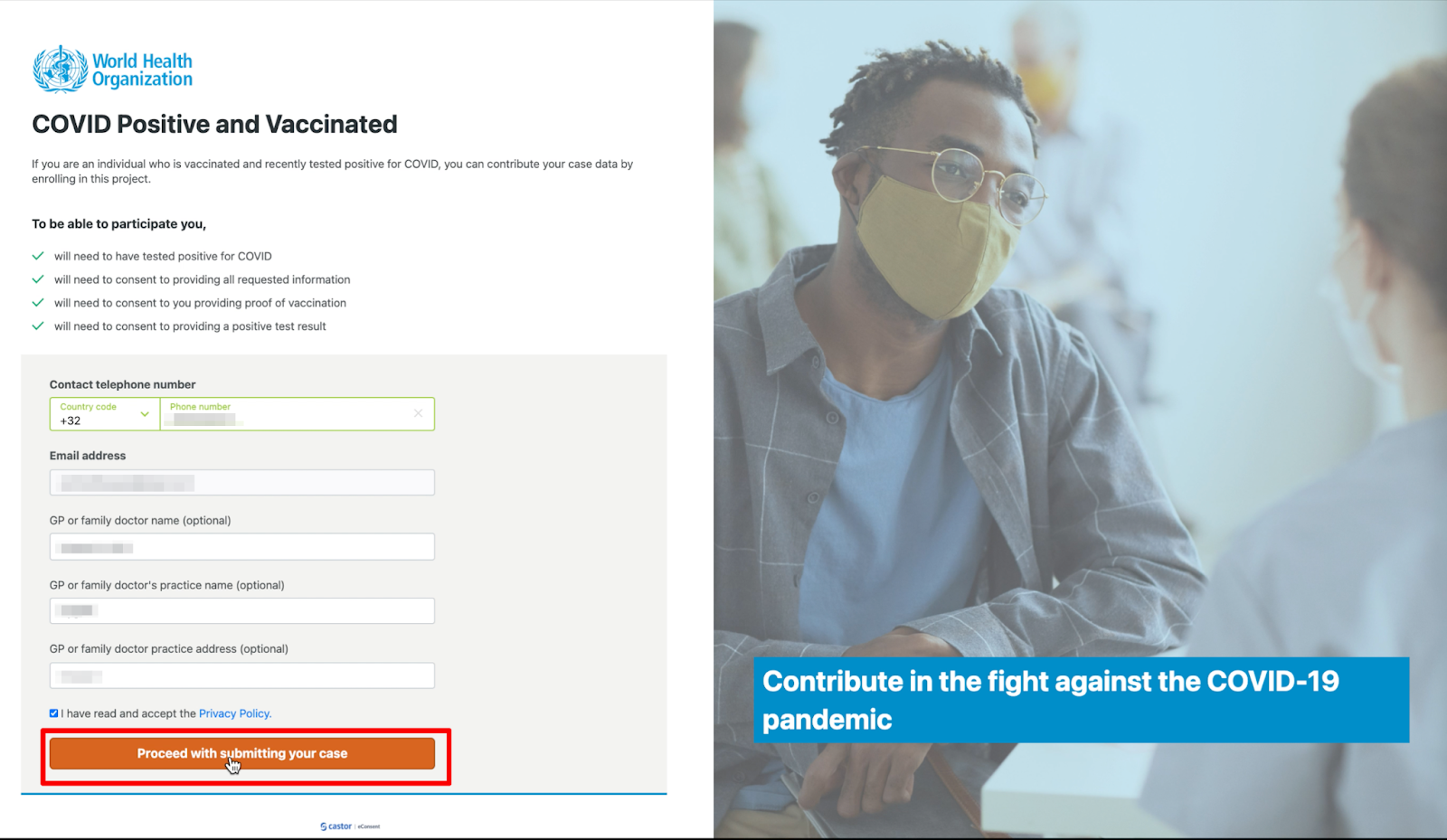
- Click on the ‘Individual’ button on the main page.
- Fill out the following details in the registration form: Contact telephone number, Email address, GP or family doctor name (optional), GP or family doctor's practice name (optional), GP or family doctor practice address (optional). Make sure to read and accept the Privacy policy.
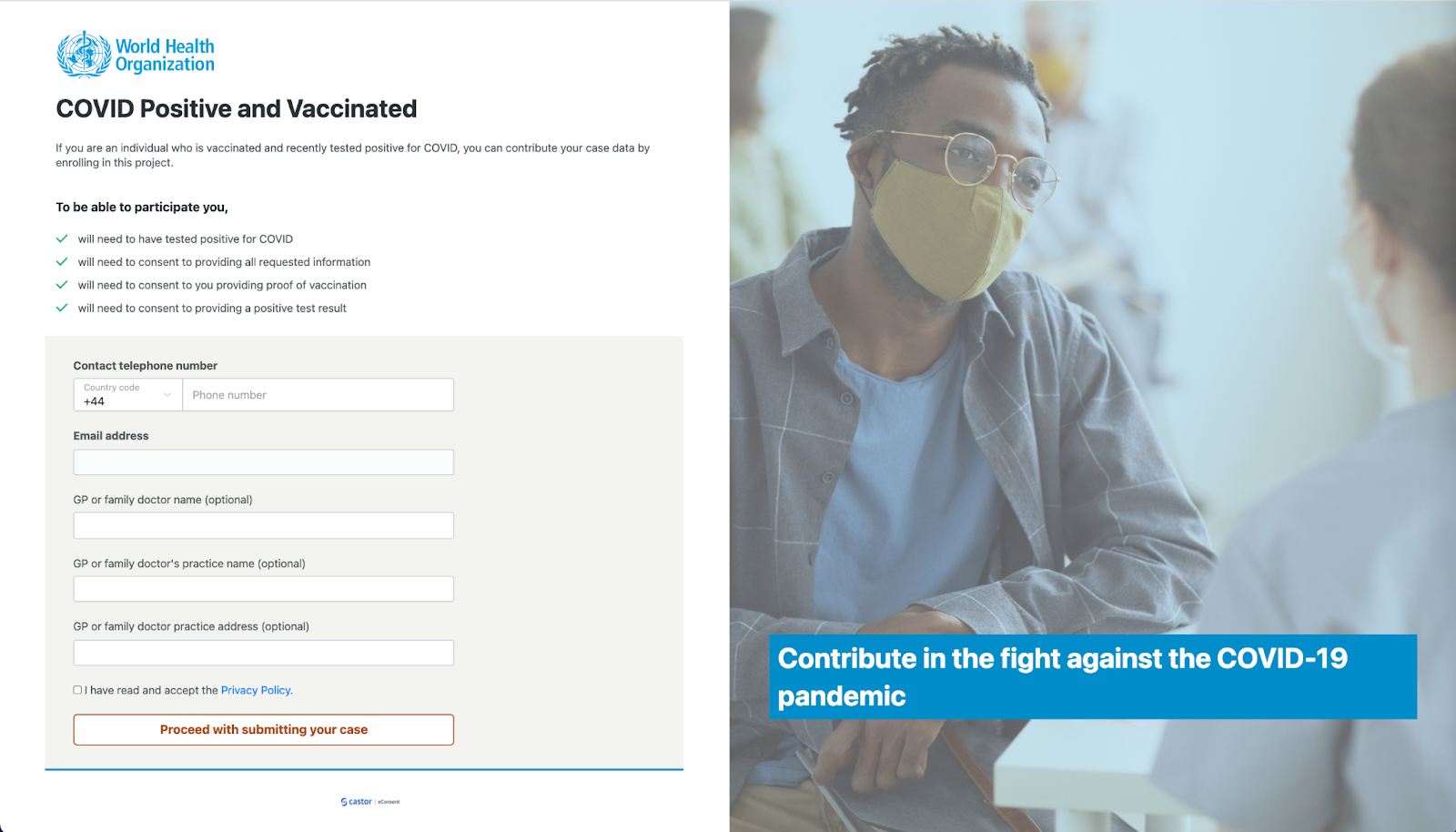
- Click on the button ‘Proceed with submitting your case’
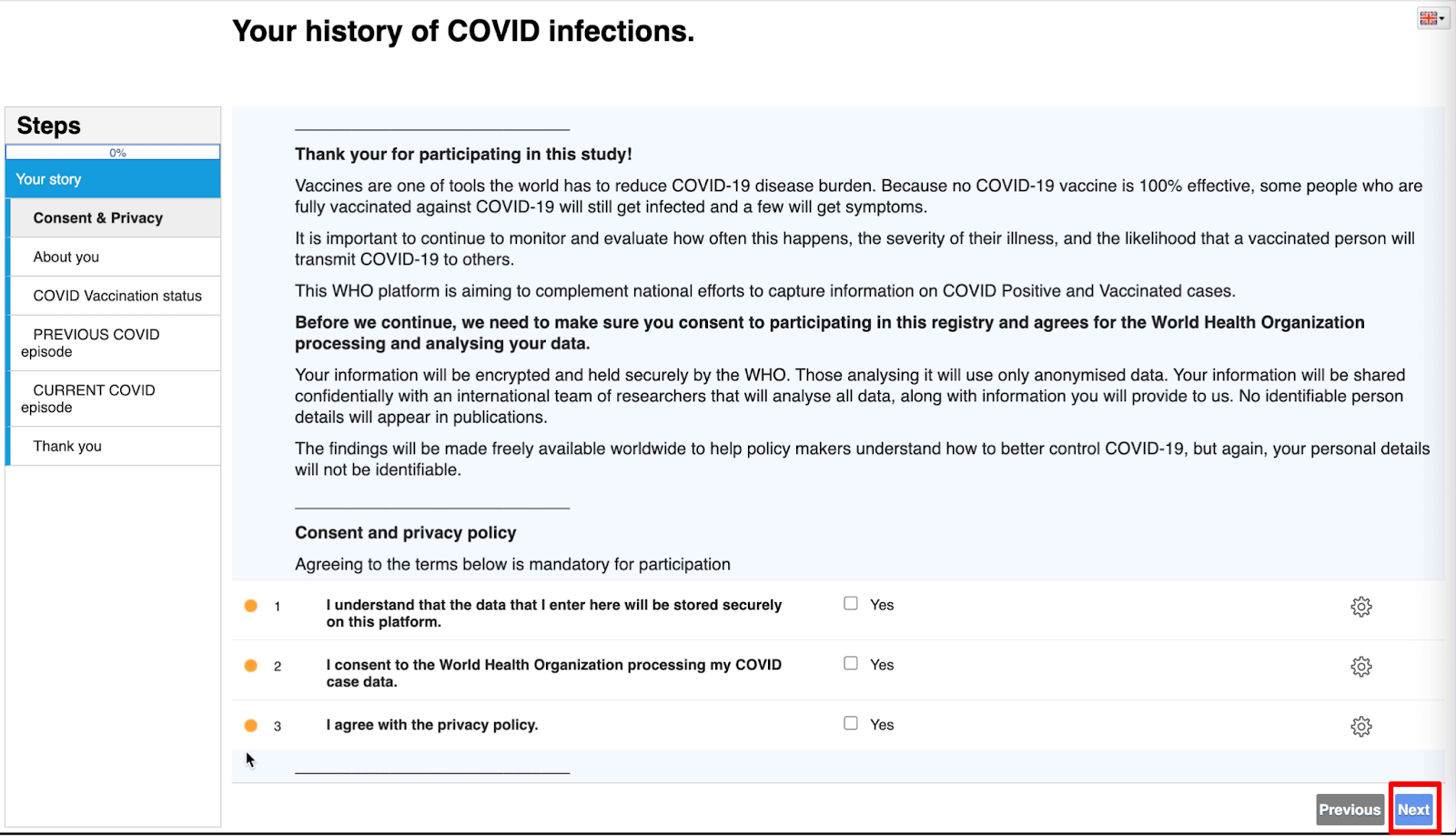
- After registration, you will be forwarded to the questionnaire.
- Please complete all the questions.
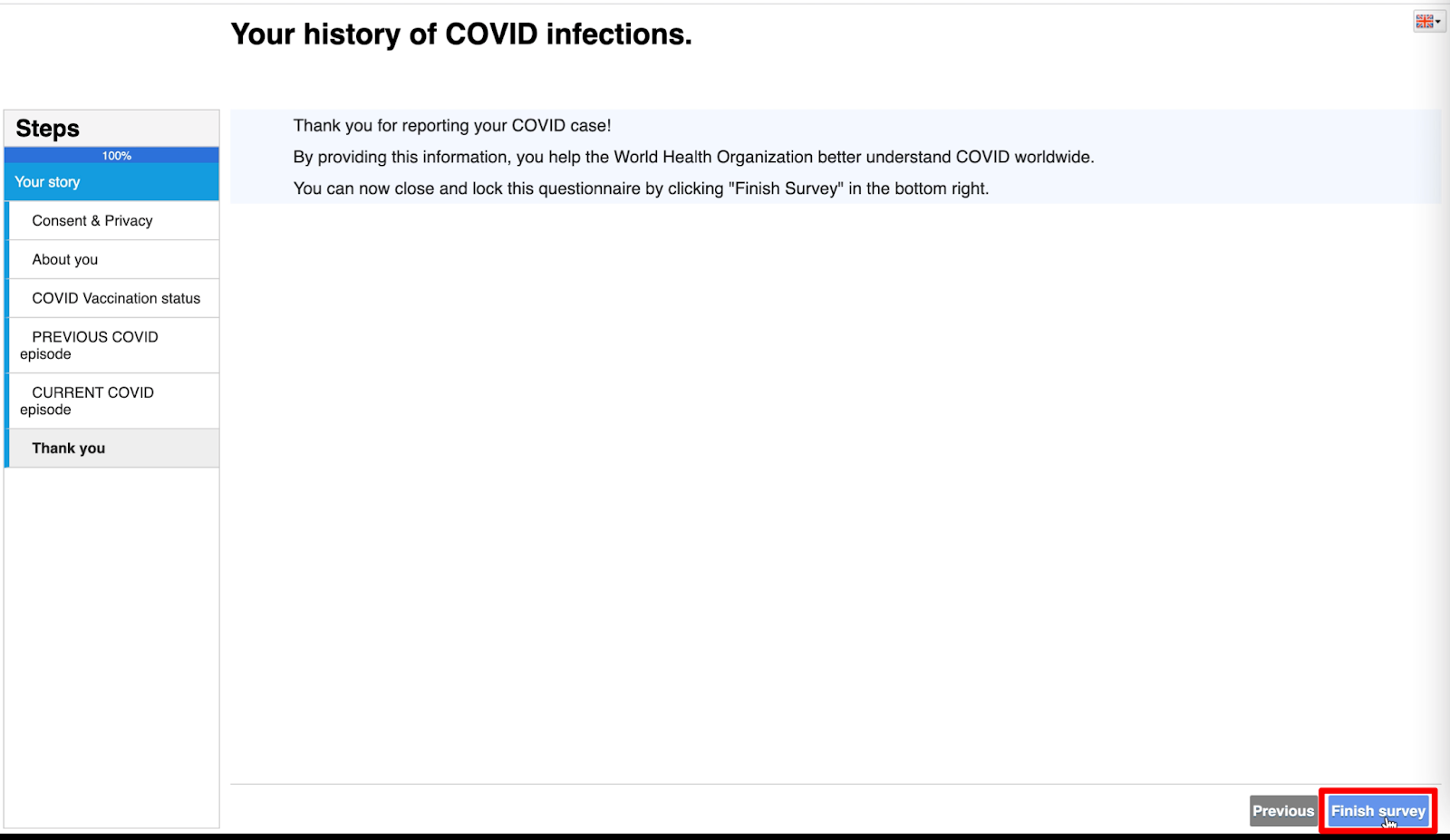
- To navigate to the next page of the questionnaire, click on the ‘Next’ button at the lower right corner.
- After you have filled out the questionnaire, please click on the ‘Finish survey’ to submit your answers.