Castor eConsent Release Notes 2022.3.x.x
Table of Contents
Major release 2022.3.0.0
Release dates:
EU server: August 30, 6 pm CEST / 12 pm EDT
US server: August 31, 10 am CEST / 4 am EDT
New features and enhancements
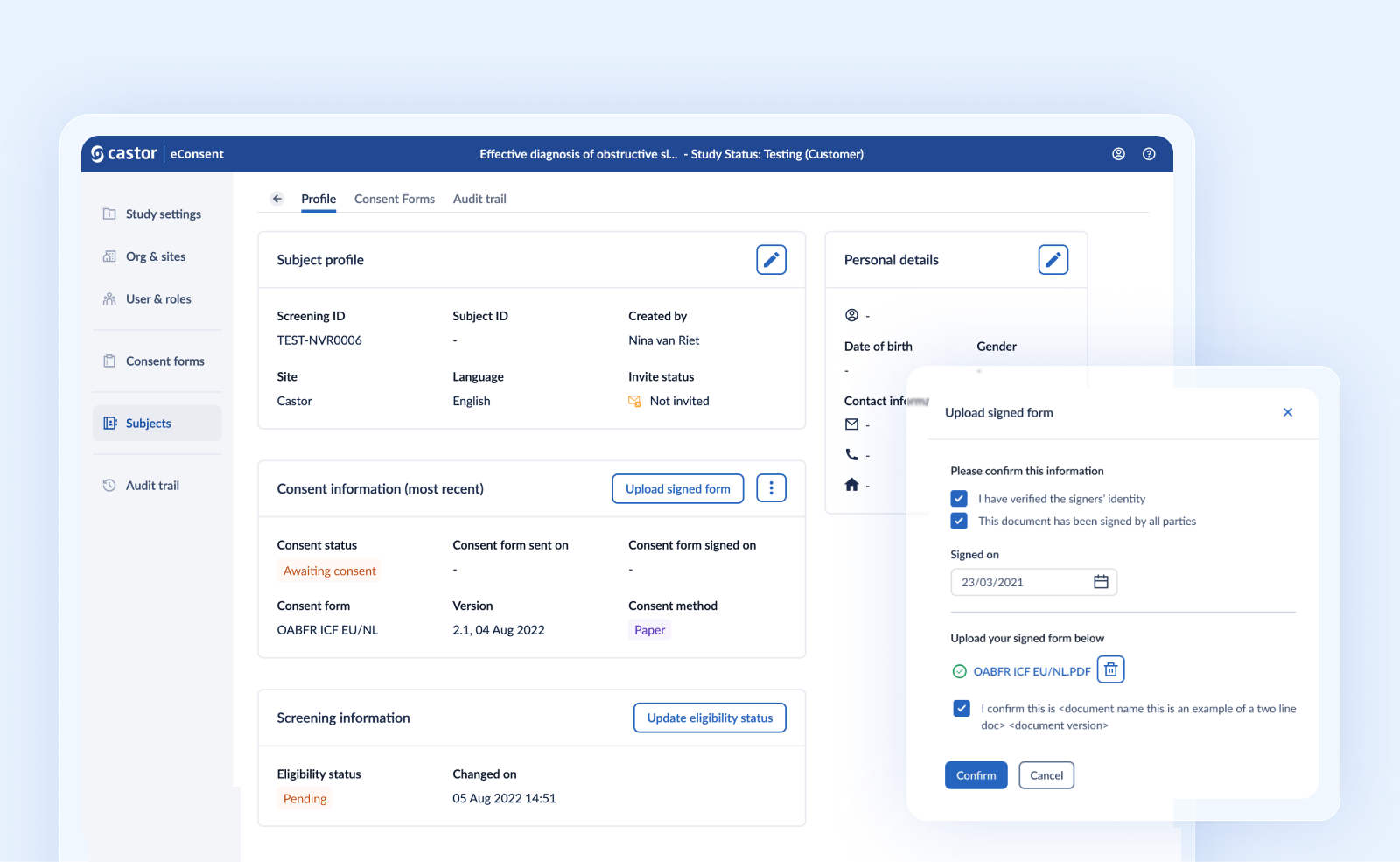
Introducing paper signatures for hybrid consent flow
We now allow the collection of paper signed consent forms for sites that cannot use e-signatures. This new feature can be enabled via the study settings, and allows you to combine multiple informed consent templates on the same study, using either an electronic signature or a paper approach to best fit your study needs.
Impact on the ICF template builder
You can define the consent method - either electronically or on paper - when creating an ICF template. If you select the ‘paper consent’ method, you don’t need to build an e-form in your template as it acts as a placeholder for storing metadata, such as name, version and language. The paper consent ICF template can be assigned to a subject once it is published.
How to manage paper signatures
The consent method - electronically or on paper - is selected when assigning an ICF to the subject. A scanned version of the paper signed ICF can be uploaded during the process if a ‘paper’ consent method was selected. The user that is uploading the file should enter the signature date and confirm that the form is signed by all parties, the signers’ identity has been verified and the uploaded file matches the ICF version.
The consent status changes from ‘Awaiting consent’ to ‘Consented’ once a copy of the paper signed ICF is uploaded. Uploaded forms can be viewed, downloaded and removed as needed. Consent status will evolve accordingly.
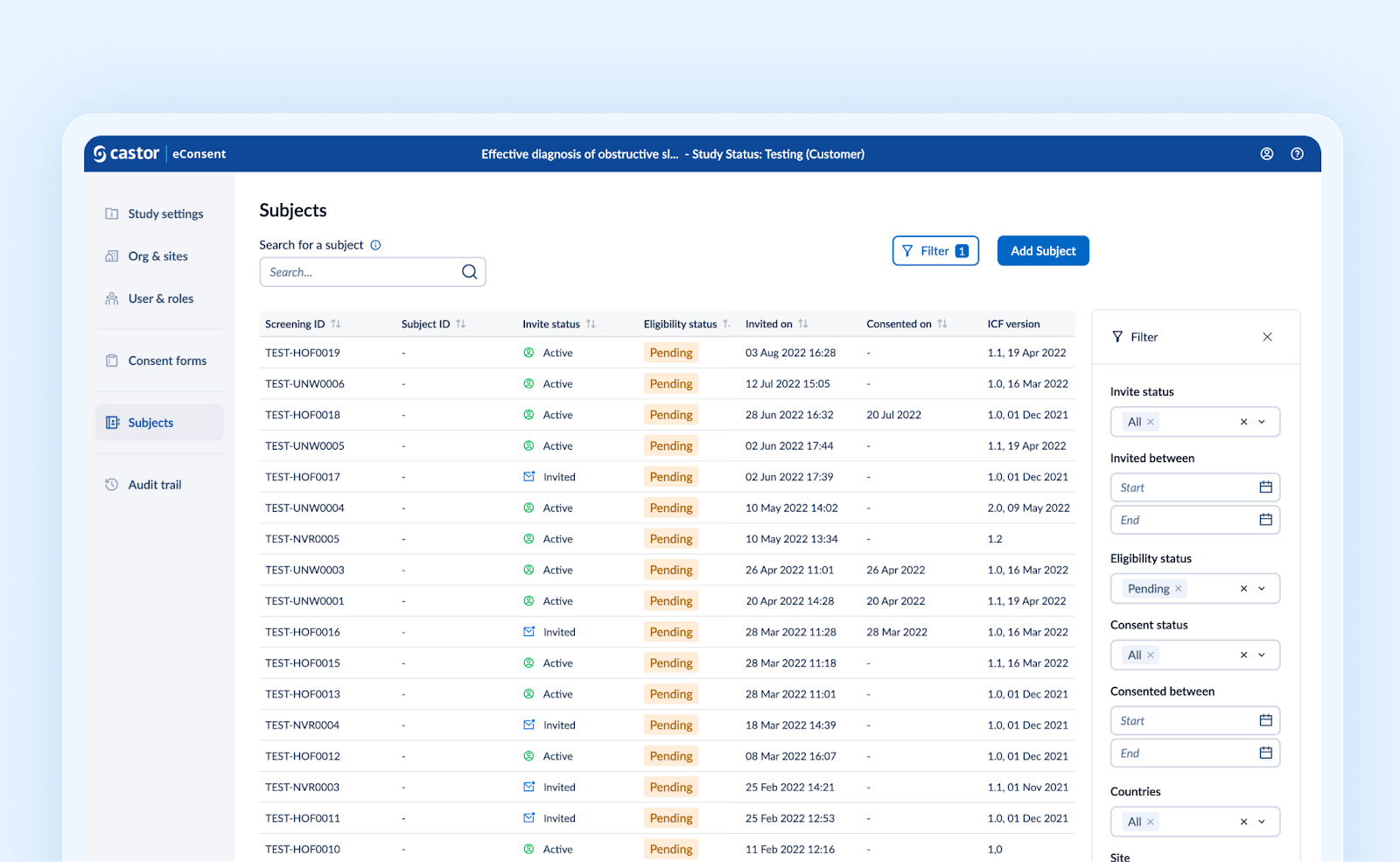
Sorting, filtering and extended searching on the Subjects overview
The Subjects overview is enriched with more capabilities to search and find the right data:
Searching on subject email address and LAR users is now supported in addition to Screening ID and Subject ID,
Subjects overview has been redesigned so you can still interact with the ‘Search’ input field while loading the results.
New filtering capabilities are added: Invite status, Eligibility status, Consent status, Country, Site, Invited between and Consented between. Archived subjects are filtered out by default.
Any applied filter, pagination and search is now persistent and remains active throughout navigation.
Sorting capabilities on the grid columns, with the exception of the ICF version, are added.
Locked ICF during the in-person signing
The in-person signing flow that allows the subject to sign (without a user account) using a site member’s tablet or computer has been improved. When opening the ICF in this mode, the ICF will now be in a locked state. Subjects cannot access other screens outside of the ICF itself without being prompted to re-enter credentials of the site staff member, ensuring security when the subject is left alone with the device.
Other enhancements
The LAR signature information and the email address of the subject are added to the ‘Informed consent information’ export report.
The general user interface is enhanced hat improve the overall look & feel.
The configurable email recipients for study notifications are now taking the receivers study permissions into account.
New entries in the Consent forms menu are now always showing at the top by default
The tasks list displayed on a subject facing screen has gone through some design improvements.
The dialog to create a new ICF template has been replaced by a dedicated screen.
The ICF template form editor now displays in read-only mode when the user has no edit permissions.
ICF languages Chinese and Japanese are no longer supported.
System defects fixes
Here’s a list of the most relevant fixes included in this release:
The email address is no longer getting pre-populated in the Last name field during user Sign up in the Chrome browser.
The subjects’ date of birth was supporting partial dates; now a date of birth should have a day, month and year.
Fixed an issue where the timezone was incorrectly impacting the version date and IRB/EC approval date on ICF templates.
The automatic logout after being idle for 20 minutes was not working as expected, this got improved.
Fixed an inconsistency issue in the 'Manage user' modal when not having edit user permissions.
Fixed an issue in the 'Value change' section for the audit trail event 'Site created'.
Fixed an issue where the user was not headed back to the previous page when hitting the 'Cancel' in the account settings page.
Fixed a styling issue on the 'Successful submission' dialog that shows after signing.
Fixed an inconsistency issue in the Subject invite email and LAR invite email.
Fixed a timezone issue related to the Version date and IRB/EC approval date fields on ICF templates.
Maintenance release 2022.3.1.0
Release date EU and US server: September 08, 10:30 pm CEST / 04:30 am EDT
System defect fixes
- Fixed an issue where changes to the ICF template after adding a LAR signature component where only visible after refreshing the page.
Maintenance release 2022.3.2.0
Release date EU and US server: October 13, 1 pm CEST / 7 am EDT
System defect fixes
- Fixed an issue where the timezone was incorrectly impacting the version date and IRB/EC approval date on ICF templates.
Maintenance release 2022.3.3.0
Release date EU and US server: October 20, 4 pm CET / 10 am EDT
System defect fixes
- The web version and the PDF version of the signed ICF are now both displaying the same timezone of the signature. The signature is displayed in the timezone of the signee.
- All the day + time fields in the subject profile are now displaying in the users' timezone.
- The signature day + time field in the 'Documents' tab on the participant facing interface is now displaying in the users' timezone.
- The 'Consented on' column is displaying the time again of the subject signature of the most recent signed ICF. (This does not include the ICFs that are signed on paper).
- The 'Send on' date in the Consent Forms grid within a subject profile was not displaying the correct date/time when having more than 1 ICF linked.