Castor eConsent Release Notes 2023.1.x.x
Table of Contents
New features and enhancements Major Release 2023.1.0.0
Release dates
EU server: March 29, 6 pm CET / 12 pm EDT
US server: March 30, 11 am CET / 5 am EDT
New features and enhancements
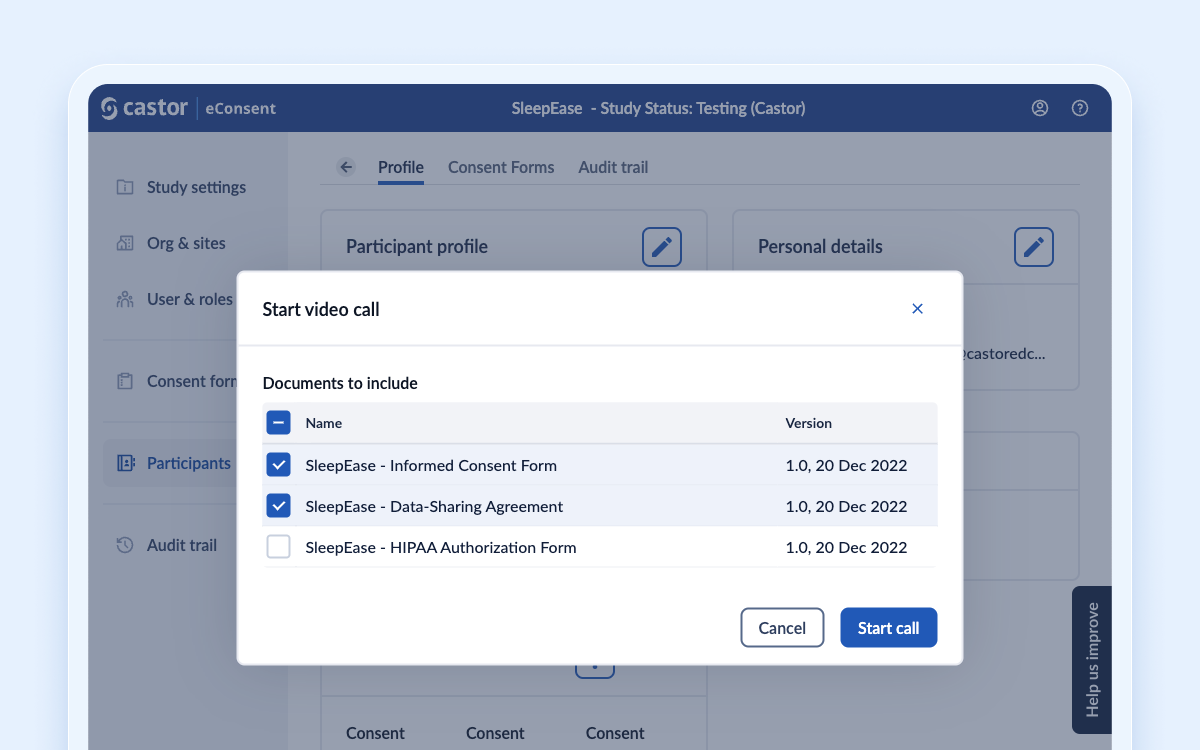
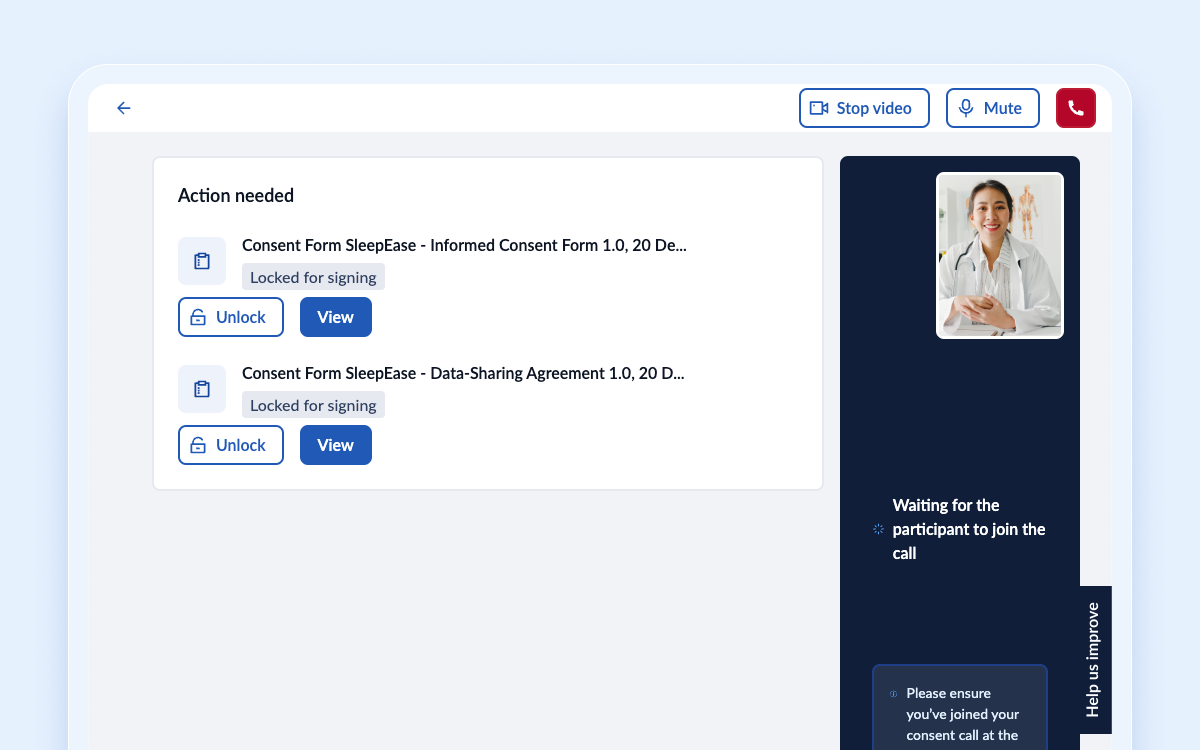
Signing multiple documents in a video call
We have improved the way study team members and participants can sign consent forms during a video call. Our new feature allows them to select multiple documents they want to review and discuss during the call with the participant. Meaning all stakeholders can now view and sign multiple documents in a single video call, streamlining the process and saving time for both parties.
To further increase control and flexibility, study team members can also lock and unlock documents, ensuring participants can only view and sign a document when it's appropriate.
Terminology
We have updated the terminology in Castor eConsent to replace all instances of the term ‘subject’ with the term ‘participant’ in line with industry standards. This term emphasizes autonomy and the active role of an individual in a study, promoting a more inclusive and respectful approach to research.
Other improvements & changes
A new column is added to the subject personal detail export which displays the Site value of the subject.
The design of the success dialog that shows after a signature was successfully submitted is improved.
When the number of participants conflicts with the Screening ID digit configuration, eConsent will now automatically continue counting with more digits. Example: 3 digit configuration would only work until the 999th participant, but now eConsent will automatically continue with 4 digits once the 1000th participant is created.
The field 'Country' has been removed from the Orgs & Sites screen and the study creation screen.
Minor change in the content of the default reminder email for signing a document.
A generic Data Intervention ATE is introduced that can be manually triggered by Castor when doing custom data interventions in a study. The ATE can contain a custom value describing what happened in the data intervention.
System defects fixes
Fixed an issue where users with edit rights for study settings would not be able to upload a study logo.
Fixed an issue where the Audit Trail would not display the ATEs of deleted subjects after manually deleting subjects from a study.
Fixed an issue where it was possible to create a participant with an archived ICF through the API.
Fixed an issue where, after switching sites, a participant's Screening ID would be reused for a new participant.
Fixed an issue where for participants with only paper ICFS the eligibility would not be updated after filling in a screening survey.
Fixed an issue where in rare cases archiving a participant would display an unrelated error message about inviting participants with paper ICFs.
Fixed an issue where inviting a participant for a second ICF would result in an error.
Fixed an issue where the 'Subject invited to sign ICF' ATE would be fired when the participant was edited.
Fixed an issue where in studies with an integration with EDC, the Participant ID with link to EDC would be created before signing any document.
Hotfix release 2023.1.0.1
Release date EU and US server: April 5, 4 PM CET, 10 AM EST
System defects fixes
- Fixed an issue where some users were not able to connect with each other in a video call.
Maintenance release 2023.1.1.0
Release date EU and US server: April 11, 8 AM CET, 2 AM EST
Improvements
- Participants that have signed in-person and never made an account will now receive an invitation to eConsent once the ICF is signed so they can retrieve their ICF.
- Minor revision in the wording of the invitation email for participants.
System defects fixes
- Fixed an issue where it was not possible to sign an ICF when using SSO.
Maintenance release 2023.1.2.0
Release date EU and US server: May 23, 8 AM CET, 2 AM EST
System defects fixes
- Fixed an issue where a user would be unable to access eConsent after closing the browser during the in-person signing flow.
Maintenance release 2023.1.3.0
Release date EU and US server: June 5, 15 PM CET, 9 AM EST
System defects fixes
- Fixed an issue where adding another ICF or sending a reminder email would change the status of a participant from 'Accepted' back to 'Invited'.
- Fixed an issue where accepting an invitation for a study team role could lead to an error in studies with a lot of participants.
Hotfix release 2023.1.3.1
Release date EU and US server: June 5, 19 PM CET, 13 PM EST
System defects fixes
- Fixed an issue where it would seem like accepting an invitation as a participant would not properly process.
Maintenance release 2023.1.4.0
Release date EU and US server: June 28, 11 AM CET, 5 AM EST
Improvements
- Improved the internal performance monitoring of eConsent.