Fill in repeating data forms for a participant
Repeating Datas can be used to register data that does not fit in the standard research protocol. A repeating data can for instance be used to register adverse events or non-scheduled hospital visits.
You can view these repeating datas in the repeating data tab of a participant:
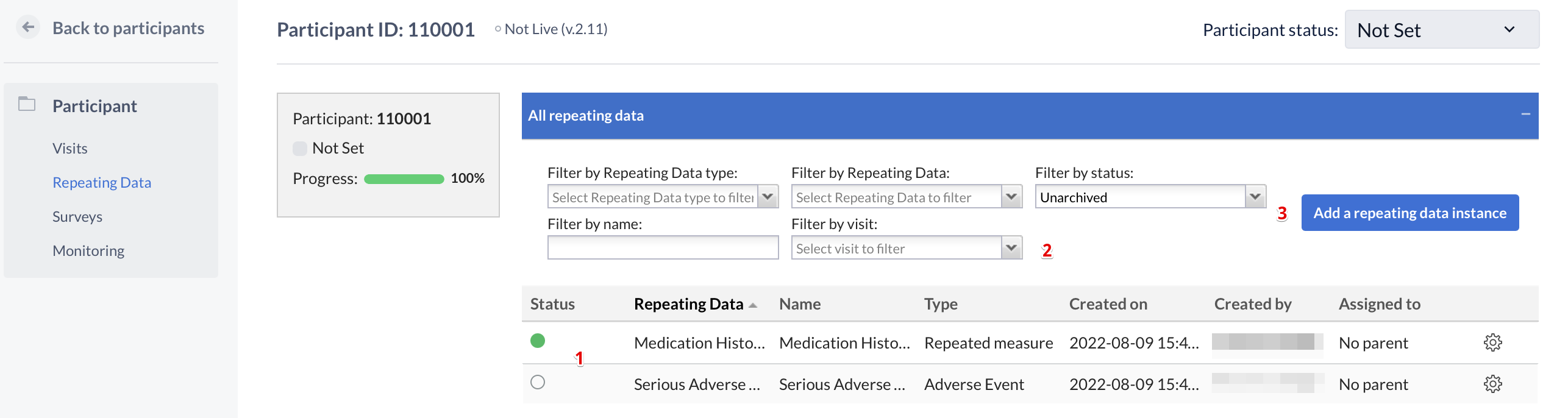
In this tab you see an overview of all repeating datas you have added to the participant (1). You can filter this list (2) by the type of repeating data (Event, Medication, Other or Unscheduled visit), the repeating data, the name of a repeating data, the visit a repeating data is connected with or the repeating data status. A new repeating data can be added with the button 'Add a repeating data' (3).
Adding a new repeating data:
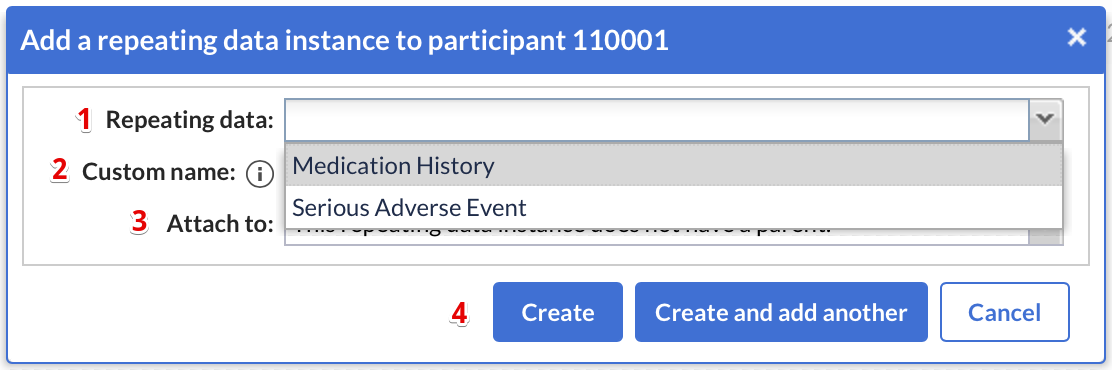
When adding a new repeating data, a dialog window will appear in which you can select:
- Repeating Data: The repeating data you are adding to the participant.
- Custom name: here you can define the repeating data name. For auto-generated names this is a preview. The name that will be actually stored may differ as it will be regenerated upon the actual save.
- You can attach the repeating data to a visit. If you choose to do this, the repeating data will show in the visits and form-navigator on the left side of the data input screen. A repeating data does not have to be connected to a visit.
- Click 'Create' to add the repeating data and return to the participant screen, or click 'Create and add another' to add the repeating data and stay in the 'Add a repeating data' window to include further repeating datas.
Creating a repeating data from the participant view:
A repeating data can also be added within the participant view. This is done by clicking the cogwheel, in the visit and form navigation, and choose 'add a repeating data to this visit':
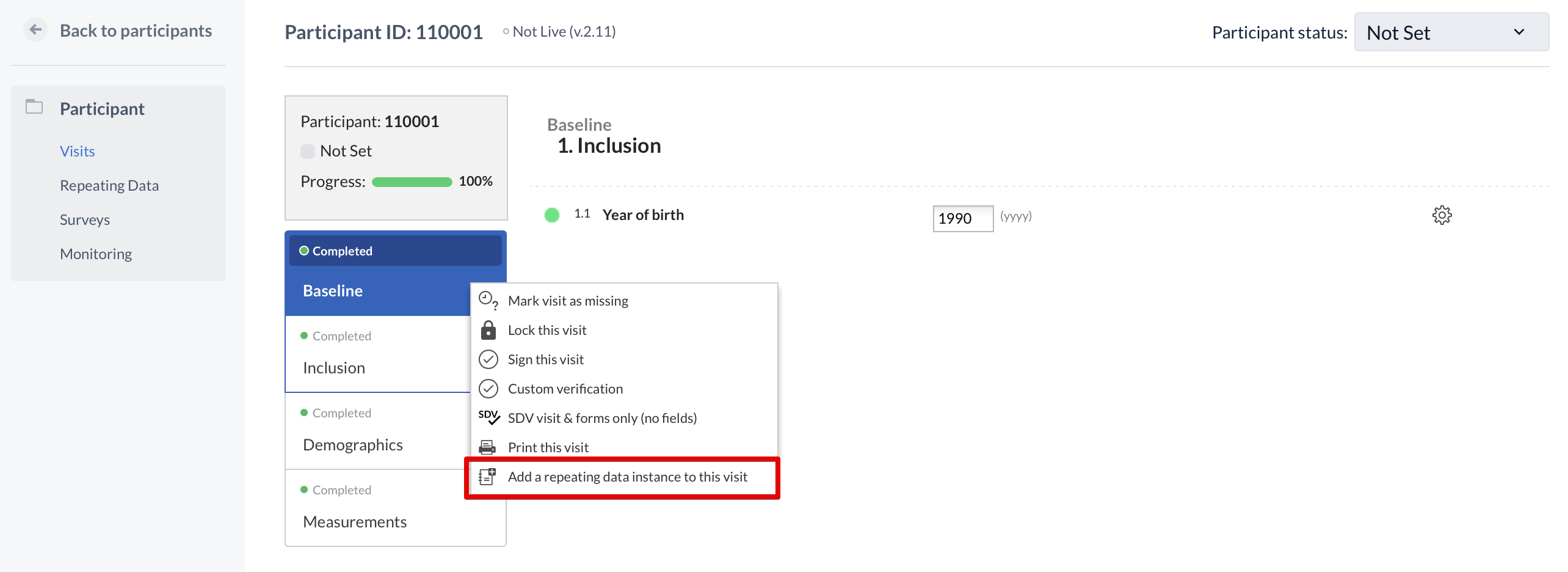
Note: A user needs 'Edit' rights to be able to create a repeating data.
Opening a repeating data:

You can open a repeating data by double-clicking on it. Here you can register data just like in a normal study form.
Repeating data options:

In the repeating data view you can click the cogwheel next to a repeating data. This will open a menu with the following options:
- Open repeating data: This will open the repeating data and enable you to add data.
- Edit repeating data: This allows you to change the details of the repeating data.
- Delete repeating data: Here you can delete the repeating data (and all data contained in the repeating data). Note: This can only be done if the study was never live!
- Archive repeating data: Allows you to archive the repeating data and all data within.
- Print repeating data: With this option you can print the repeating data with all forms and gathered data.