Release notes Castor SMS februari 2020 - versie 2020.3
For English see below
Dinsdag 11 februari 2020
Aanpassingen:
- Notificaties:
- De volgende e-mail variabelen zijn toegevoegd aan de gebeurtenis "Monitorvisite toegevoegd":
- Uitvoerende monitor - {{ executing_monitor }}
- Visite datum - {{ visitDate }}
- Type monitorvisite - {{ visitType }}
- De e-mail voor gebeurtenis "Studie goedgekeurd RvB" kan nu ook verstuurd worden naar gebruikers met een bijbehorende deelnemende afdelingsrol.
- De volgende e-mail variabelen zijn toegevoegd aan de gebeurtenis "Monitorvisite toegevoegd":
- Instituten menu:
- Een nieuw veld is toegevoegd waarin de website van het instituut vermeld kan worden. Middels het link-icoon kan wordt de pagina in een nieuw browser tabblad geopend.
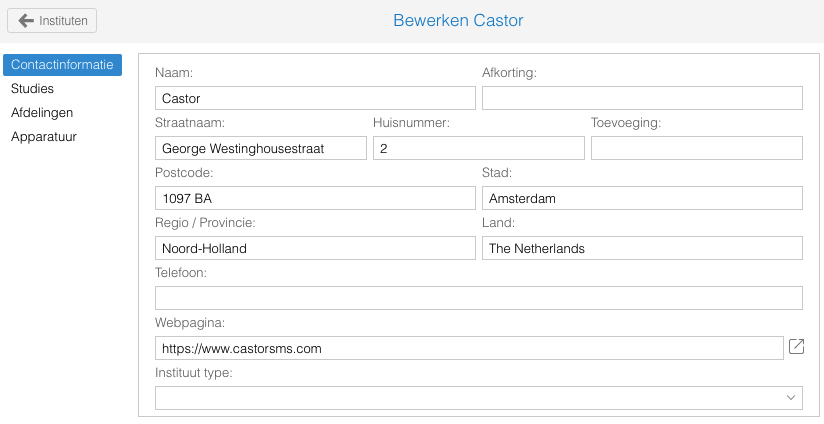
- Een nieuw veld is toegevoegd waarin de website van het instituut vermeld kan worden. Middels het link-icoon kan wordt de pagina in een nieuw browser tabblad geopend.
- Templates voor brieven:
- De volgende variabelen zijn toegevoegd en vanaf nu beschikbaar om gebruikt te worden:
- Externe toegang EPD nodig - ${externalEprAccess}
- Datamanagement systeem - ${dataManagementSystem}
- Type proefpersonen - ${testSubjectsType}
- Plek opslag studiedocumenten tijdens uitvoer - ${documentStorageLocation}
- De volgende variabelen zijn toegevoegd en vanaf nu beschikbaar om gebruikt te worden:
- Account registratie: De Terms of Use moeten vanaf nu door nieuwe Castor gebruikers actief geaccepteerd worden middels een checkbox.
- Dashboard: Een filter voor Status is toegevoegd aan de Lijst weergave voor Amendementen.
- Documenten: SPSS bestanden (.dat, .sav en .sps) worden vanaf nu ook ondersteund.
Tuesday 11th of February, 2020
Changes:
- Notifications:
- The following email variables are added to event "Monitor visit added":
- Executing monitor - {{ executing_monitor }}
- Visit date - {{ visitDate }}
- Monitoring visit type - {{ visitType }}
- The email for event "Study submission board accepted" can now also be send to user(s) with a related participating department role.
- The following email variables are added to event "Monitor visit added":
- Institutes menu:
- A new field is added to collect the web page of the institute. The page can be visited by using the link-icon; a new browser tab will open.
- Field label "House number" is changed into "Street number"

- Templates for letters:
- The following SMS variables are added and available for use:
- External EPR access required - ${externalEprAccess}
- Data management system - ${dataManagementSystem}
- Type of participants - ${testSubjectsType}
- Document storage during study - ${documentStorageLocation}
- The following SMS variables are added and available for use:
- Account registration: From now on, new Castor users must actively agree with the Terms of Use.
- Dashboard: A filter for Amendment status is added in the List view for Amendments.
- Documents: SPSS format files (.dat, .sav, and .sps) are also supported for file upload.