Release notes Castor SMS July 2020 - version 2020.14
For English see below
Woensdag 15 juli 2020
Aanpassingen:
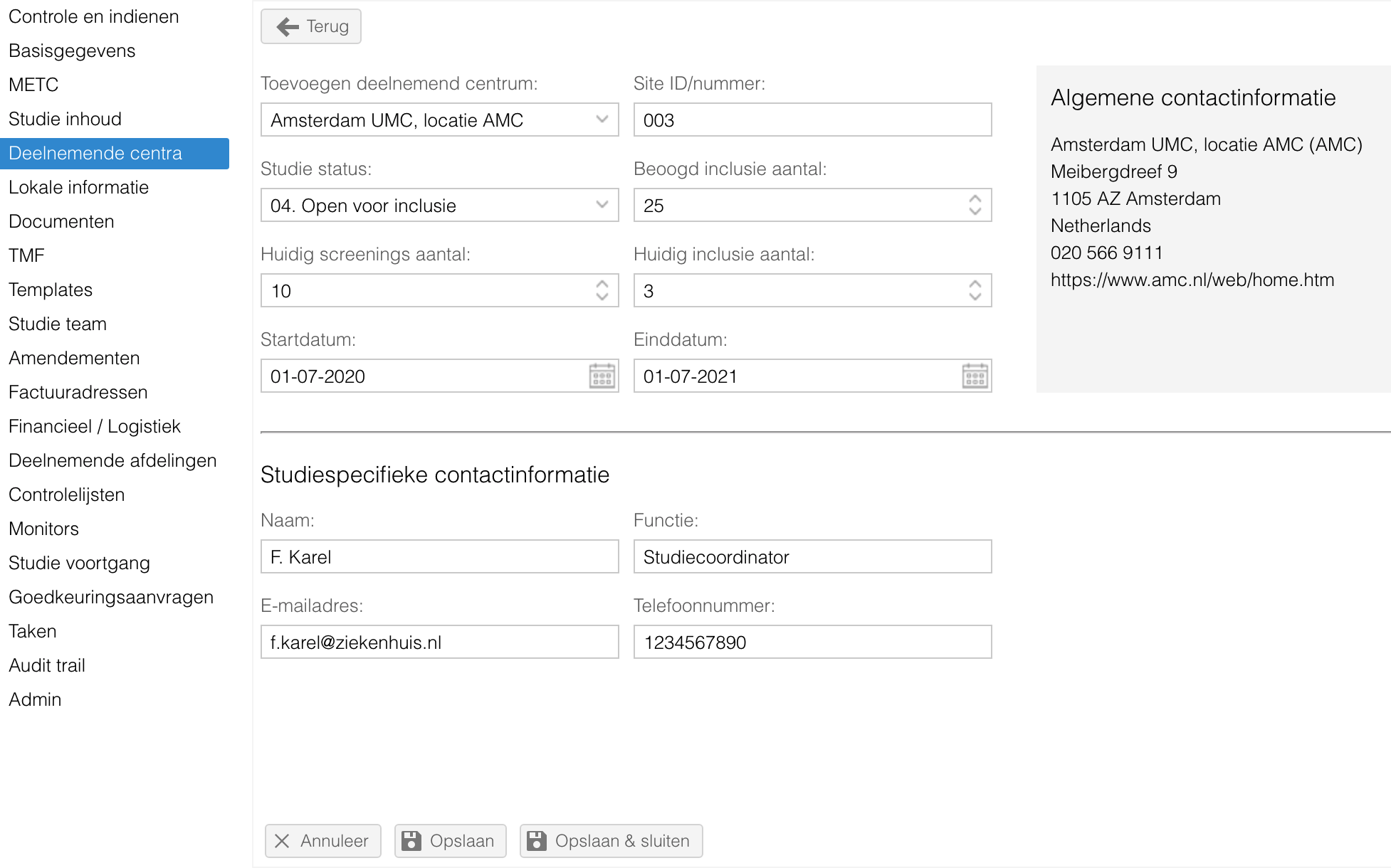
- Bij de deelnemende centra is het nu mogelijk om een studiespecifiek contactpersoon toe te voegen. Tevens is de algemene contactinformatie inzichtelijk.
- Elektronische handtekening:
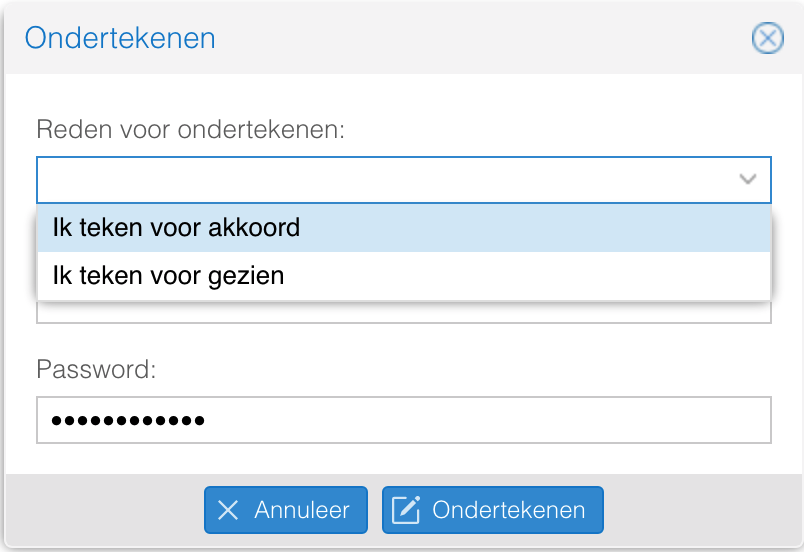
- Bij het ondertekenen van een goedkeuringsaanvraag wordt om een reden gevraagd.
 Na ondertekening wordt de reden ook zichtbaar bij de digitale handtekening.
Na ondertekening wordt de reden ook zichtbaar bij de digitale handtekening. - Gebruikers wordt gevraagd om zowel een e-mailadres als wachtwoord in te vullen als zij een goedkeuringsaanvraag ondertekenen. Per gebruikerssessie wordt eenmalig naar het e-mailadres gevraagd.
- Bij het ondertekenen van een goedkeuringsaanvraag wordt om een reden gevraagd.
Wednesday July 15th, 2020
Changes:
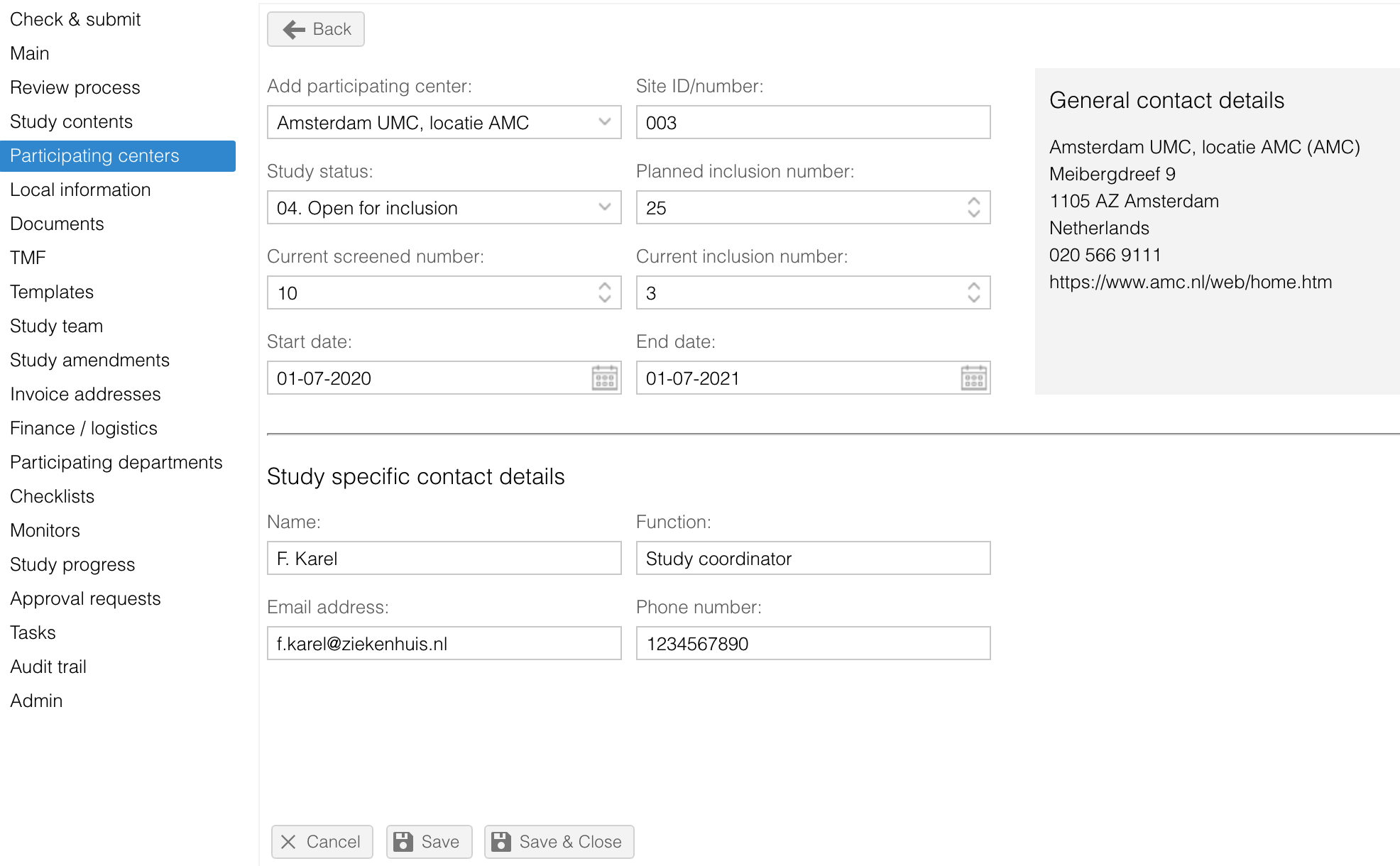
- Contact details per study site can entered. The general contact information per site is also available.
- Small improvements are made to the digital signature to :
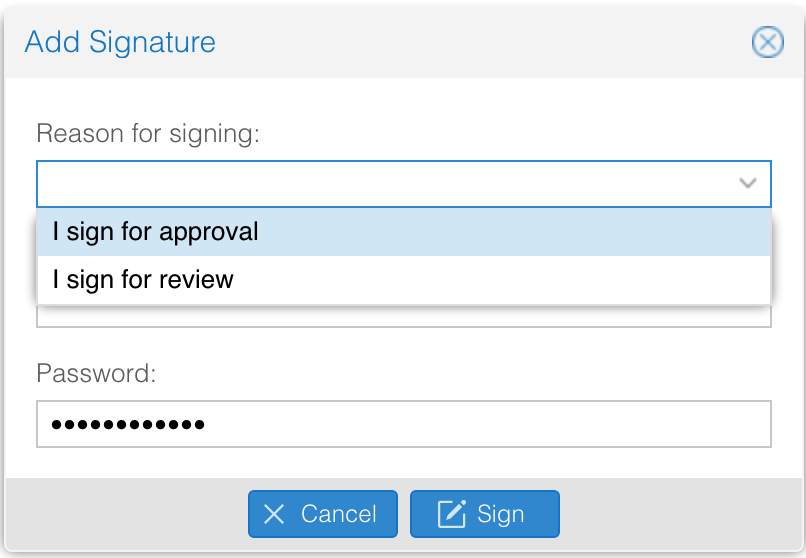
- By signing an approval request, the user is asked to give a reason.
 This reason is showing in the signature statement.
This reason is showing in the signature statement. - Users are now asked to provide email and password the first time they sign a step or phase during their session. After the first signature, only password is required.
- By signing an approval request, the user is asked to give a reason.