Release notes Castor SMS september 2019 - versie 2019.18
Dinsdag 17 september 2019
Aanpassingen:
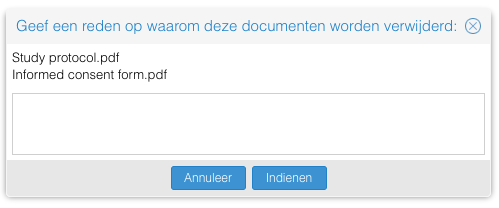
- Documenten: Het definitief verwijderen van studiedocumenten kon eerst alleen één voor één. Nu is hier een bulk actie voor toegevoegd waardoor meerdere studiedocumenten in één keer definitief verwijderd kunnen worden.
- Als voorbereiding op de integratie met Castor EDC wordt het 'Studie voortgang' tabblad nu ook getoond bij studies die nog niet door de RvB zijn goedgekeurd.
- Audit trail: Het audit trail event 'Gebruiker geopend' is toegevoegd voor wanneer een gebruiker vanuit het 'Gebruikers' menu wordt geopend.