The 'Upload file' field in CDMS
The 'Upload file' field allows users and survey participants to upload files up to 5 MB in size. It is possible to upload larger files (up to 2GB per file) by purchasing the Large File Upload feature for your study. All file types are accepted, with the exception of executable files and SVG image files.
Please note that at the moment, it is only possible to upload one file at a time into the ''Upload file'' field.
Here is an example of the 'Upload file' field, shown in a study form:
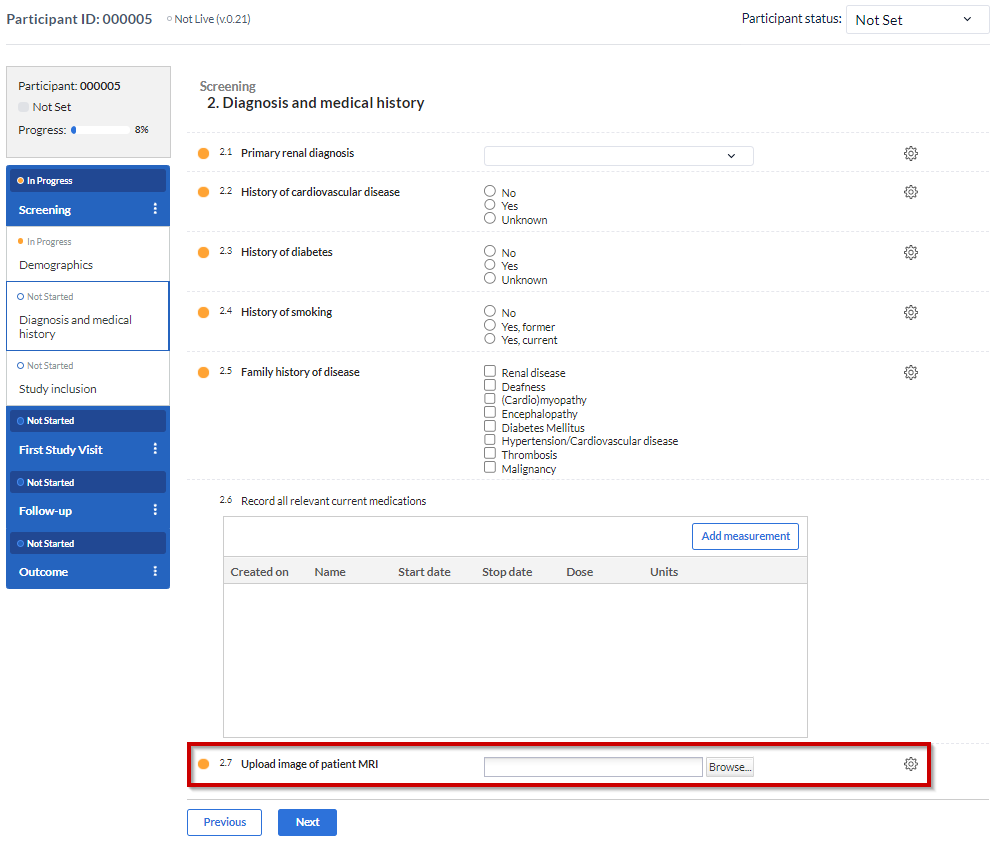
Once a file has been added to the field, a download button is shown below the file, with which the uploaded file can be downloaded. For image files and PDFs, there is also the option to view the file without downloading:
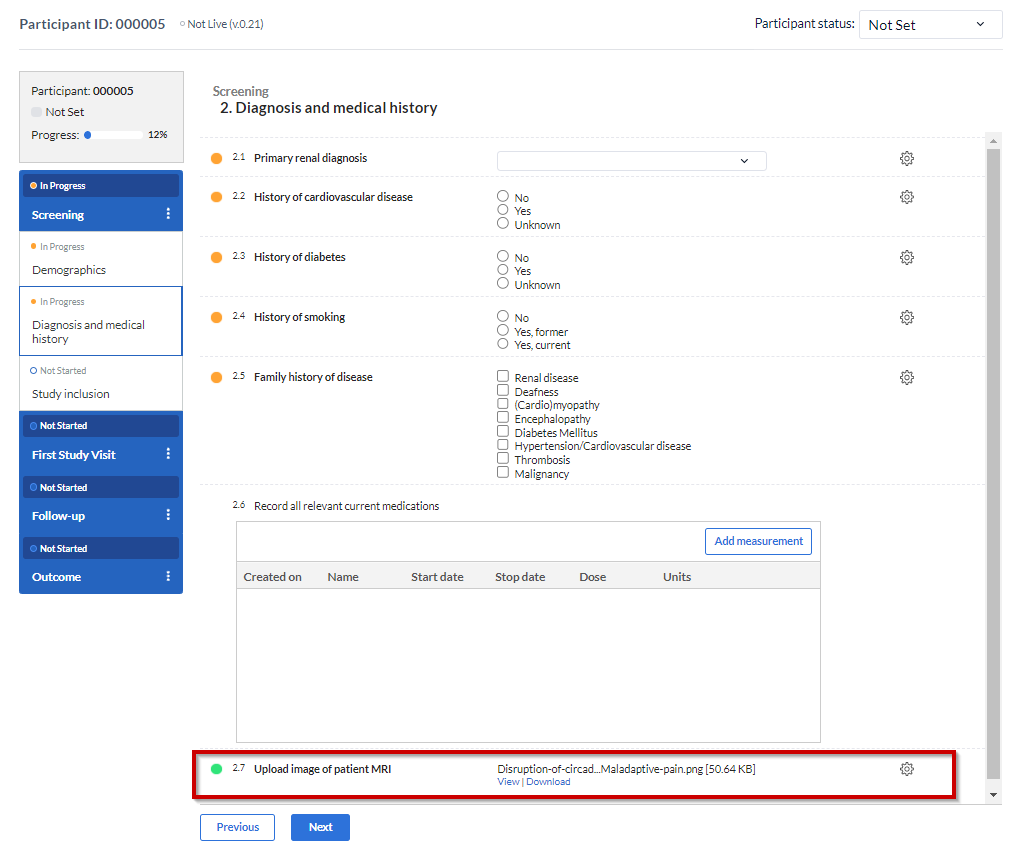
If the uploaded file needs to be removed or changed, you can delete the file using the cogwheel beside the field - select 'Delete upload' to remove the file. Once cleared, you can add a new file.
Please note:
It is possible to download files with 'View' rights only. You can download the files by exporting the data study from Castor. You will find the steps to do this by visiting the page How to export study data in CDMS.