Het indienen van een studie
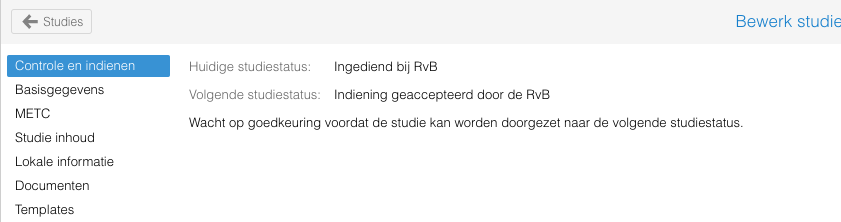
Het indienen van een studie, bij bijvoorbeeld het wetenschapsbureau of raad van bestuur, gaat middels het tabblad "Controle en indienen". Hier wordt de huidige status van de studie weergegeven en welke verplichte velden en documenten aangevuld moeten worden.
Het indienen van een studie voor lokale goedkeuring:
- Ga naar het Studies menu;
- Open de studie;
- Ga naar het Controle en indienen tabblad;
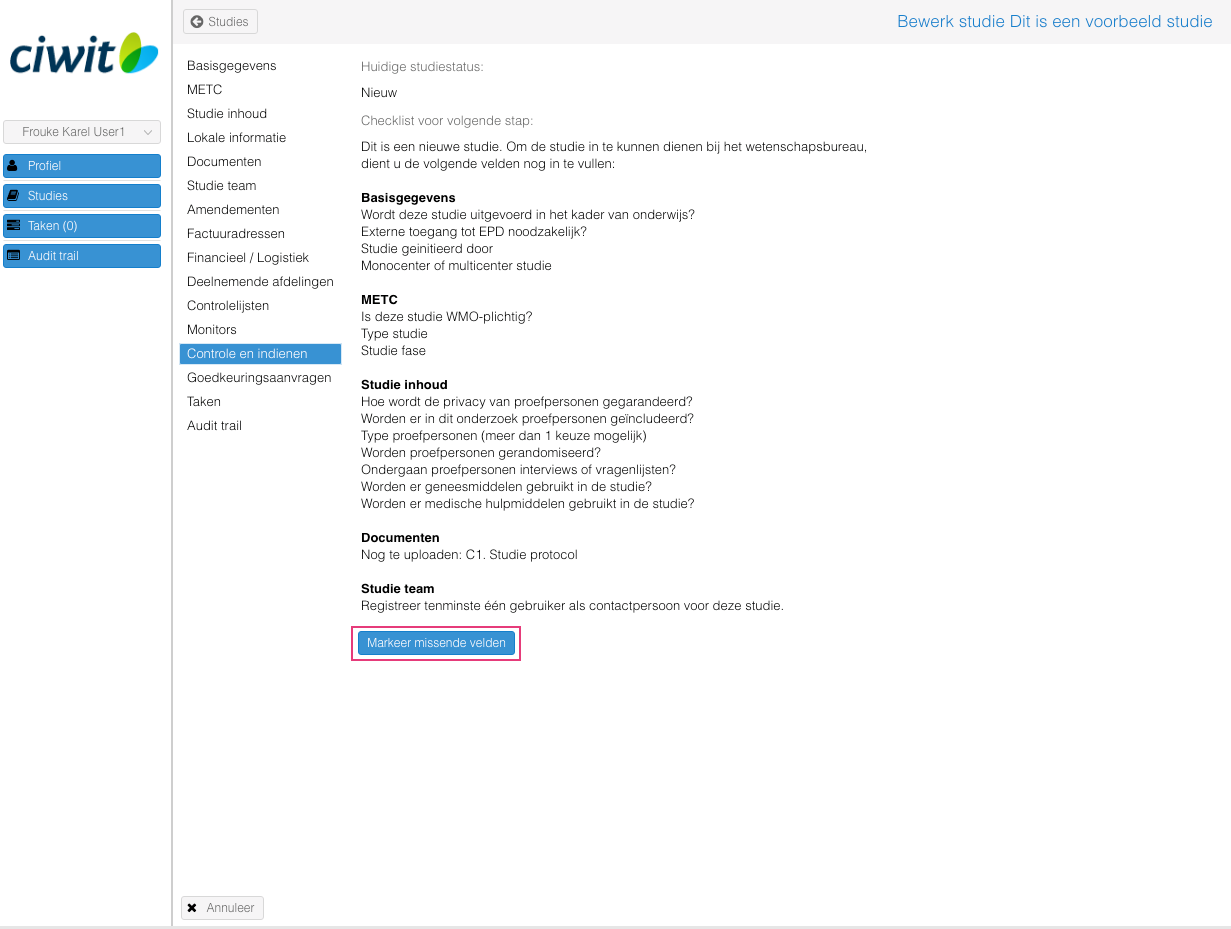
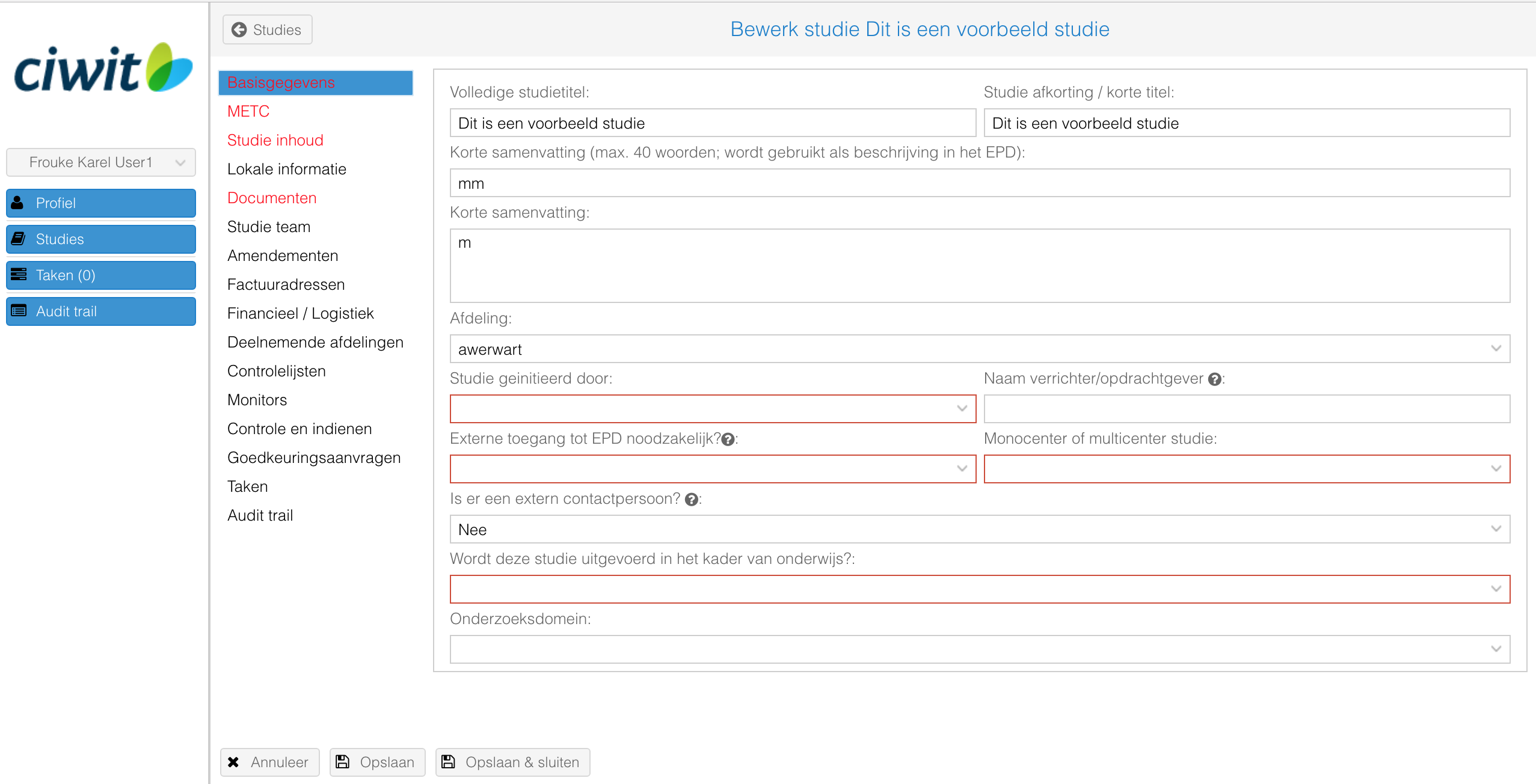
- Indien nodig: vul de verplichte velden in en upload de verplichte typen documenten (gebruik de "Markeer missende velden" knop om de verplichte velden rood te markeren);
- Klik op de "Studie indienen" knop.
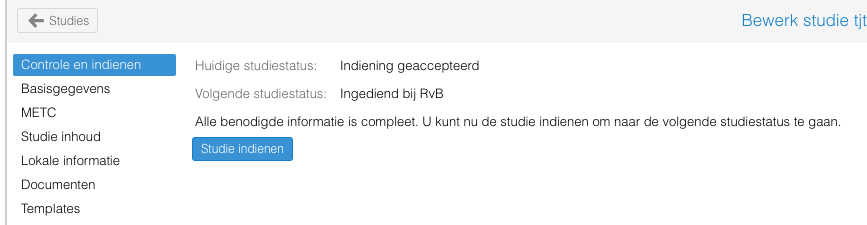
De studie is ingediend.