Import sites in CDMS
Table of Contents
Users with ‘’Manage Settings'' rights can bulk create sites in CDMS by creating a CSV file that they can upload.
Importing Sites
To do that follow the below steps:
1.Navigate to the Settings>Study option
2.Click on the ''Manage sites'' button
3.Click on the ''Import files'' button 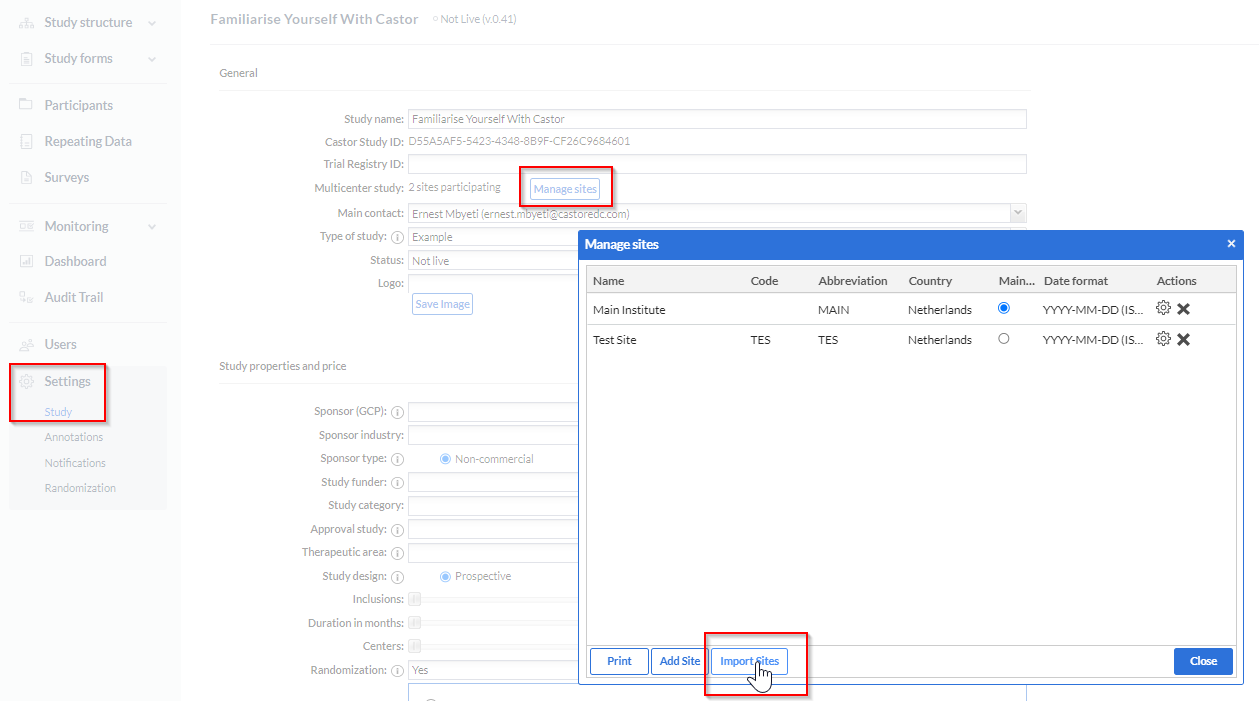
4.In the window that will open, choose your file and click on ''Upload file''
To facilitate the import creation process, a sample file is available for download in the CDMS import dialog by clicking on ''Download Sample File''.
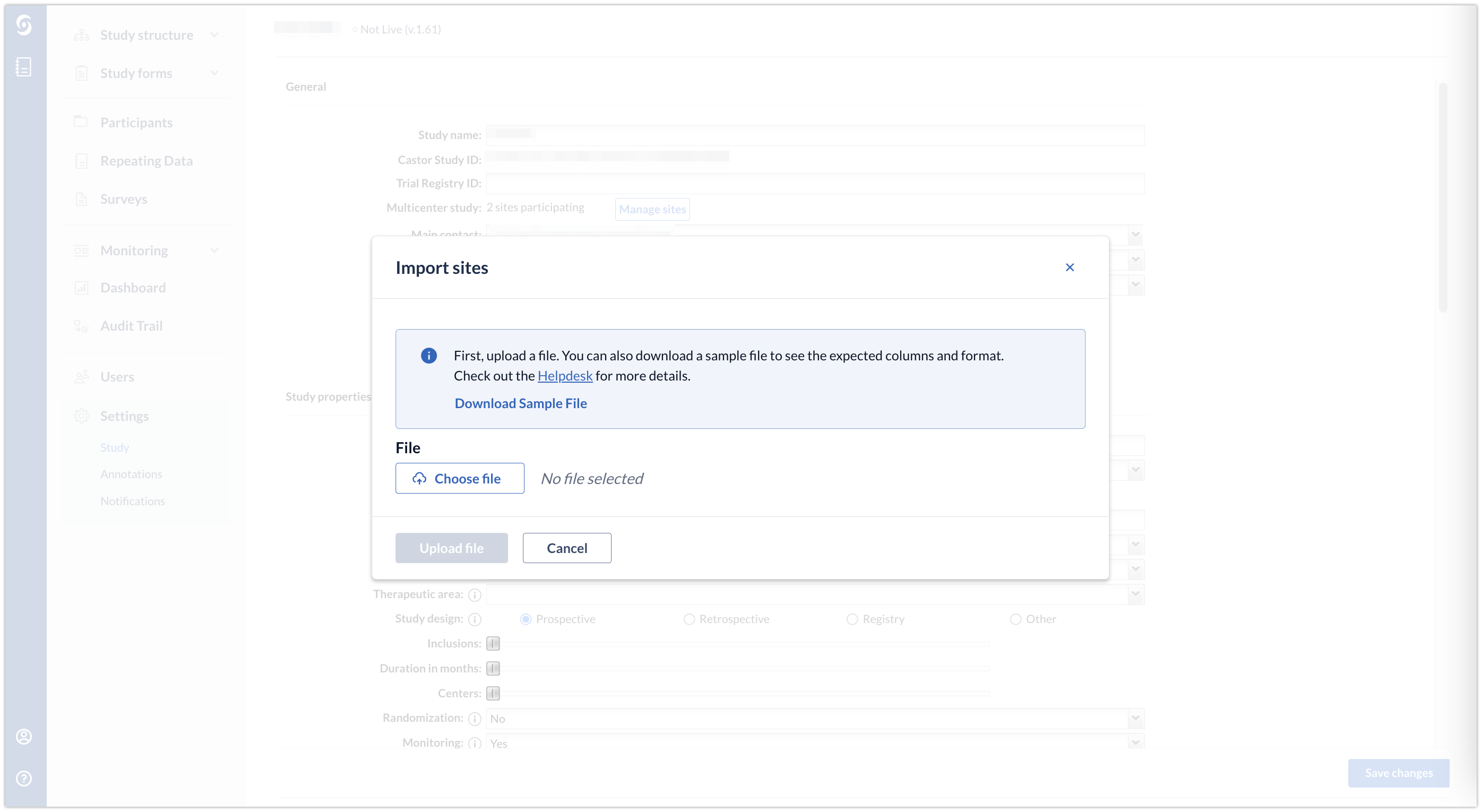
Example
Using the sample file we can bulk add sites to the study: 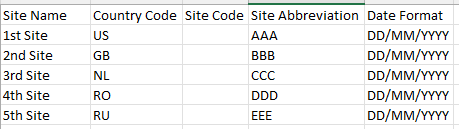
- Site Name: Enter the name of the site
- Country Code: 2 letter format, (e.g. NL as in The Netherlands - full list here)
- Site Code: Enter the code of the site. The code must consist of 3 alphanumeric characters or left blank.
- Site Abbreviation: Enter the site abbreviation
-
Date format: Enter the date format that will be applicable for the site. The date format has to be one of the available formats that can also be found from the dropdown menu of the ''Manage sites'':
- YYYY-MM-DD (ISO)
- DD-MM-YYYY
- MM/DD/YYYY
- DD/MM/YYYY
- Month, DD, YYYY
- DD Month YYYY
- DD-MMM-YYYY
- YYYYMMMDD
In order to edit and import the Downloadable Sample File please follow the instructions that can be found on the ‘How to prepare an Excel file to be imported as CSV' article.