Submit an approval request to a study
Please note - notifications can only be managed by system administrators.
When to use approval requests
An approval request can be used when a specific person or department needs to approve a study related issue, for example a lawyer, financial controller, privacy expert, project manager or head of department. Approval request options are linked to user roles, which also managed by Admins.
By default, users with the ‘User’ role can only view the ‘Approval request’ tab from within a study, but this role cannot add approval requests
Approval requests and participating departments
Some institutes are using both participating departments and approval requests. Adding both separately is a lot of work. In this cases, it is possible to automatically add (inactive) approval requests when participating departments are added.
- This is a separate setting and can be turned on/off by Admins.
- Admins can link a participating department to an approval request from the Approval Requests tab in Settings.
- When the settings is turned on and there is a participating department linked to the approval request, the approval request will automatically be created when the participating department was created. It will be in an inactive status, therefore it still has to be submitted.
Submit a study approval request
- Go to the 'Studies' tab.
- Open a study.
- Go to the 'Approval requests' tab.
- Click on 'Add approval request'.
- Fill in the form.
- Click on 'Save' to only create the approval request, or click on 'Save & Submit' to submit the request. A notification will be send.
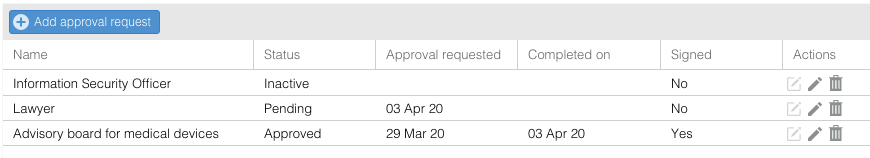
Once created, users with the related approval request role can view this study and can edit the approval request. Depending on the notification settings, the users may also receive a notification regarding the added approval request.
Sometimes, timelines can be short. In those cases, it is useful to send an approval request directly to someone you already discussed the study with. It is possible to send an approval request to a specific person with that approval request role.
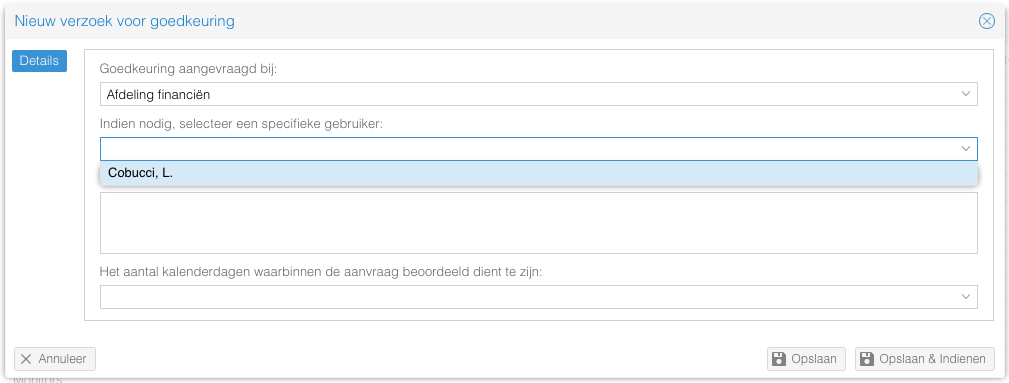
- This question is hidden by default. Please send a request to support@castoredc.com to get it enabled for you.
- Only the users with the accompanying approval request role will show in the drop down. It is not possible to send it to users that do not have the role.
- Other users with the approval request role will also gain access to the study, like normal. However, they will not receive a notification email.