Using the Castor survey compliance listing
Table of Contents
Surveys compliance report
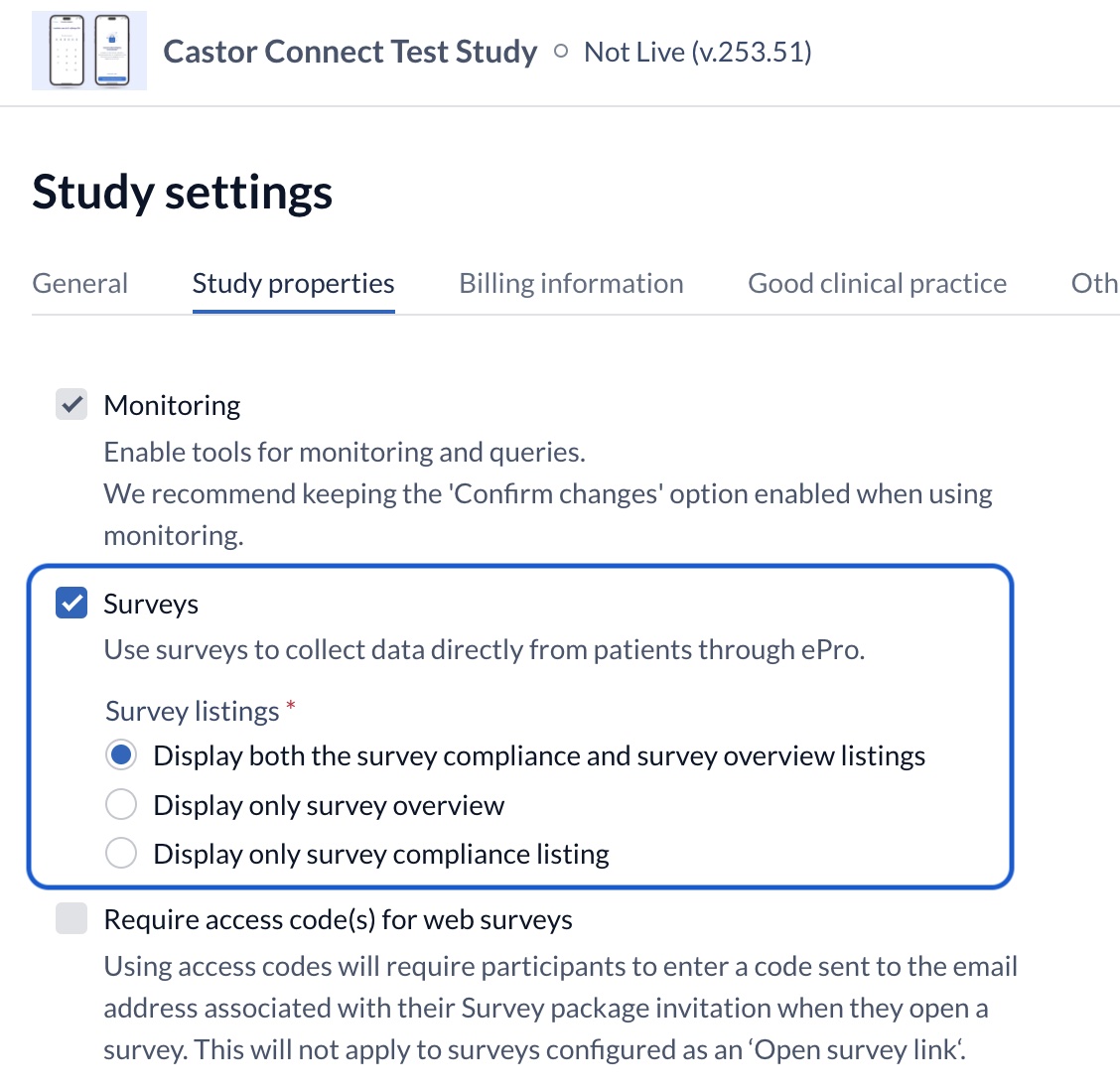
As of release 2024.1.0.0, studies with surveys turned on will be able to use our new survey compliance listing. This listing will become available by default for all users with required permissions, allowing them to monitor the compliance (or survey completion rate as a %) of their participants.
What is the Castor survey compliance listing?
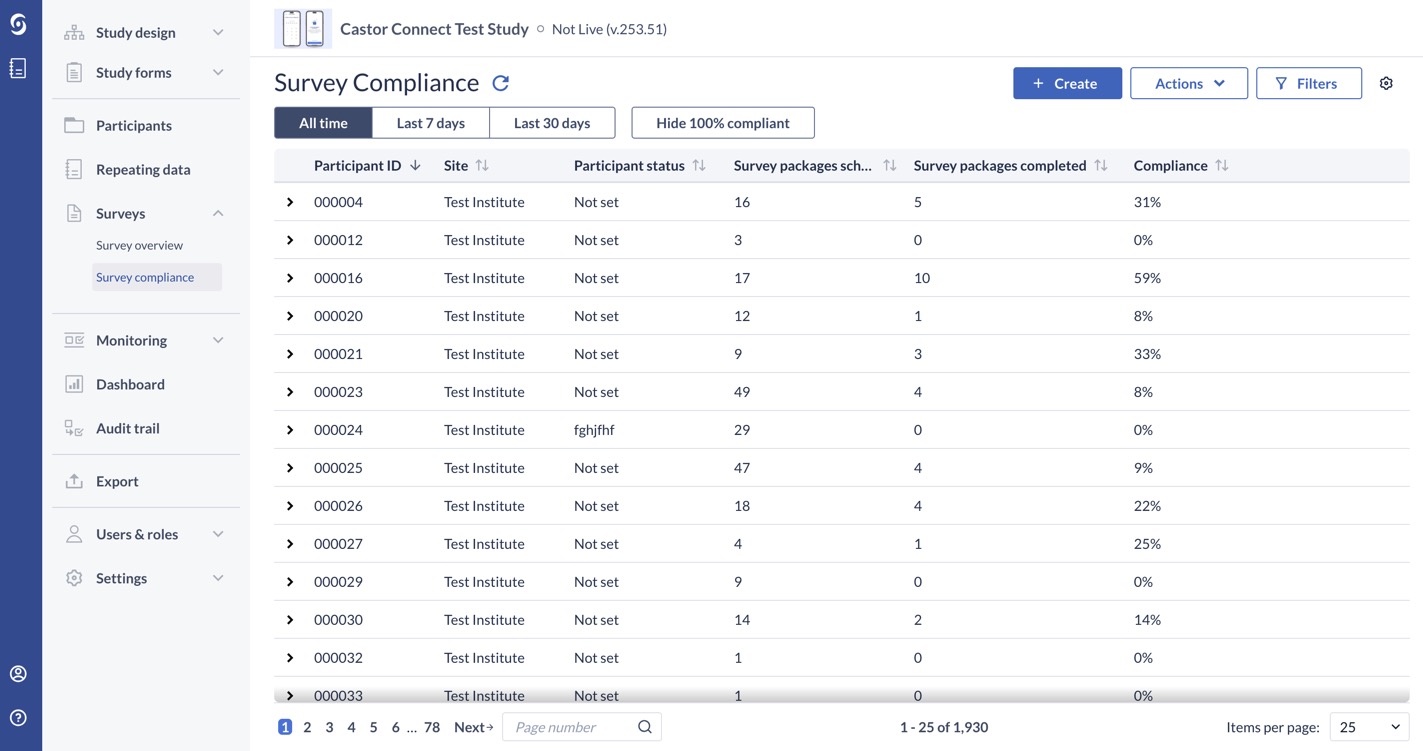
The new listing includes a list of participants a user has access to, as well as a ‘sub-grid’ or secondary list of survey package invitations associated with that participant. By default this will be for ‘all time’ and display a compliance percentage. This percentage reflects how many survey package invitations have been completed out of all that have been made available for that participant. As you apply filters to the data, the compliance percentage will update to reflect these.
Which filters are available?
A range of filters are available for your use, with archived participants and surveys hidden/not included by default:
- Site
- Participant ID (exact)
- Participant Status
- Compliance %
- Availability window (date to date)
- Parent
- Survey package name
- Survey package status
- Completion method (Web or Castor Connect)
-
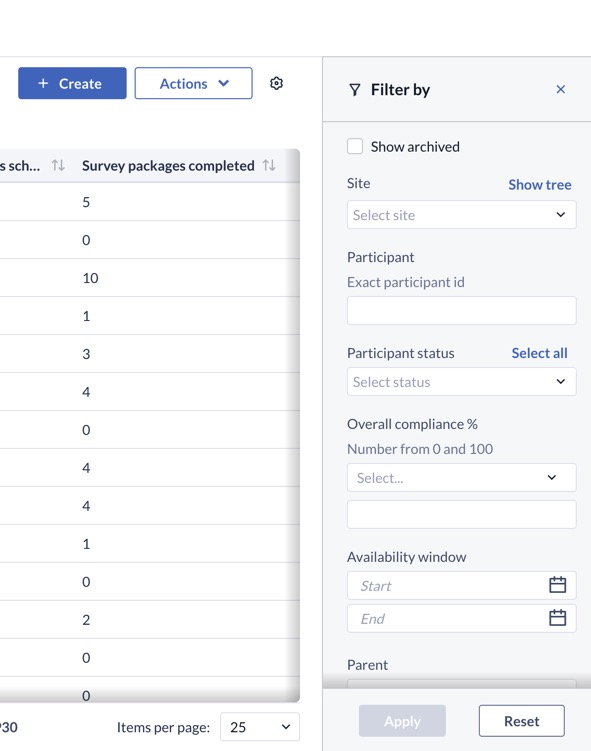
Can I save my filters/views of the data?
Users can hide or show various columns in the listing at both the participant and survey package invitation level. Any updates to the displayed columns and filters will be saved in your browser, allowing you to navigate away or log-out from the platform and return without losing your personalized view.
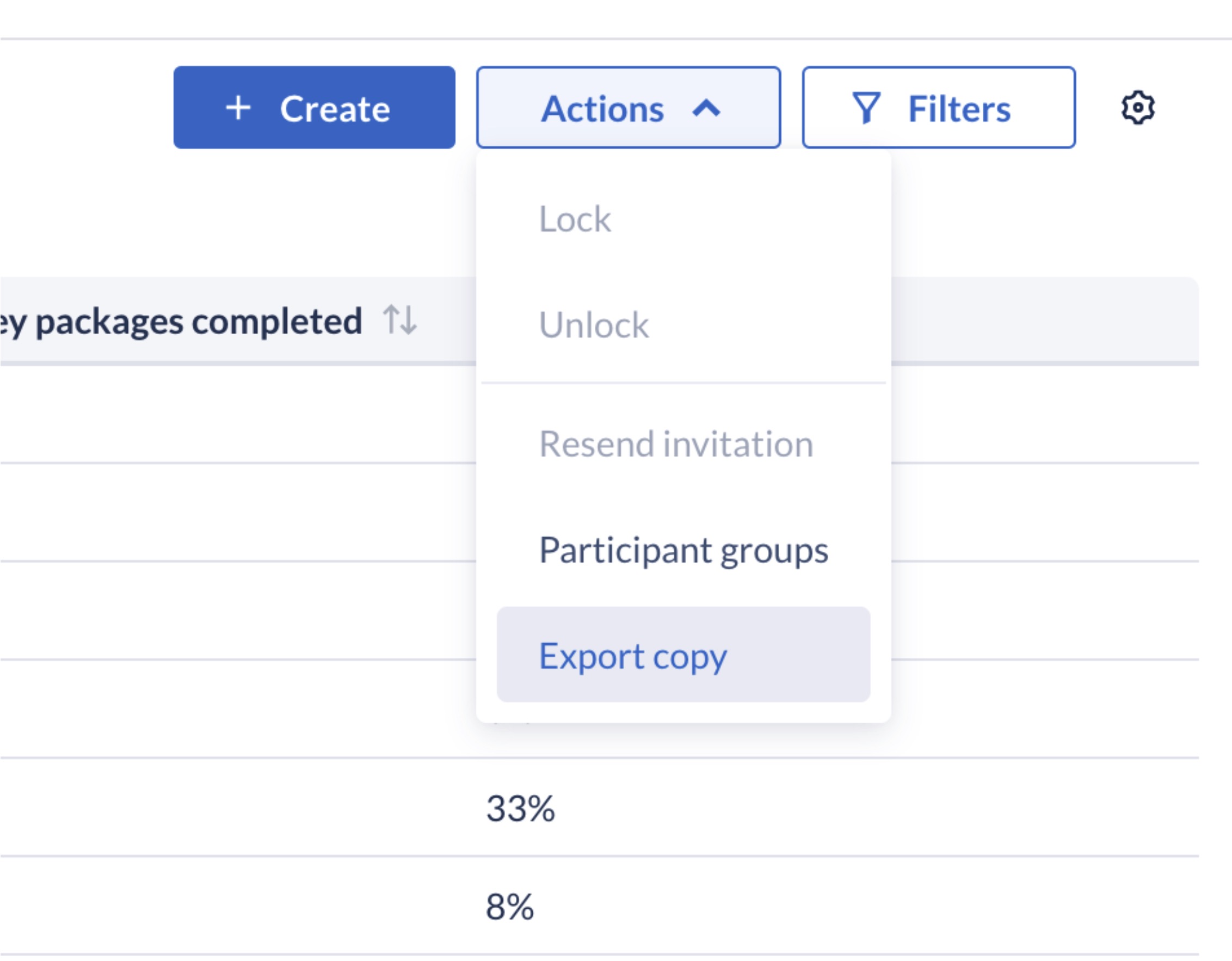
Can I export a copy of the data?
Users with permissions to both view survey data and export data from the platform will be shown an ‘Export’ button from within the listing view.
What happens to the existing listing?
The more we discussed and gathered feedback on how to make survey management more effective - the more we learned that our users were already effectively using the existing survey listing and data exports to develop or inform compliance reports outside of our platform.
We developed the new listing to meet this need, but know that there are some studies for whom this won't work because of their study configuration or more specific study needs. For this reason, the pre-existing listing will continue to be available - and can even be configured to remain your only standard survey listing from within study settings.