Create a Repeating Data Form in CDMS
Table of Contents
Repeating Data study structure can be used to register adverse events (AE) and also to record any other data which is not part of the main study protocol, for instance any unexpected hospital visits or emergency surgical procedure.
Creating Repeating Data is almost the same as creating Visits - in the article below, you can learn about how to create a Repeating Data structure.
Create a Repeating Data structure
Navigate to the 'Structure' tab and then to 'Repeating Data' to open the Repeating Data structure editor. Click the '+ Add' button to create a new Repeating Data:
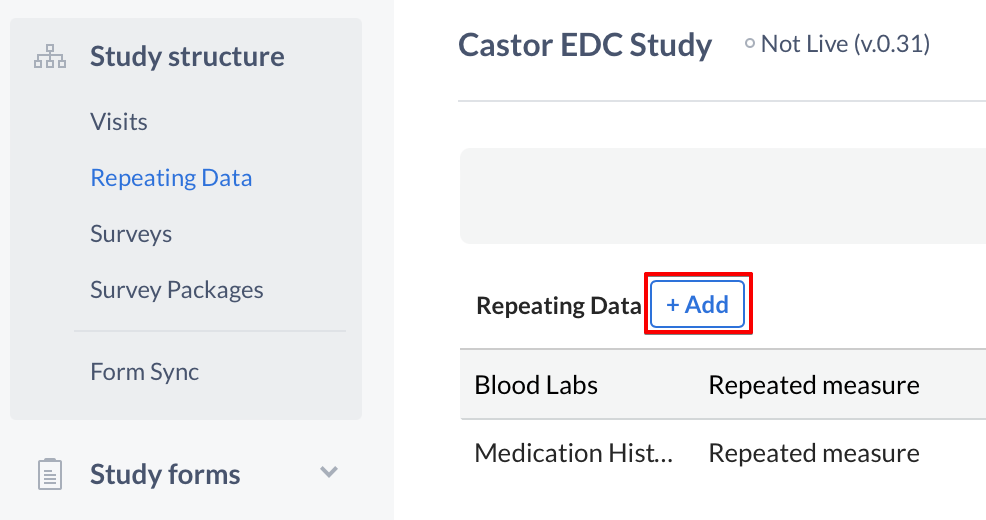
The 'Add a Repeating Data' dialog window will open in which you can enter details about the Repeating Data you will create:
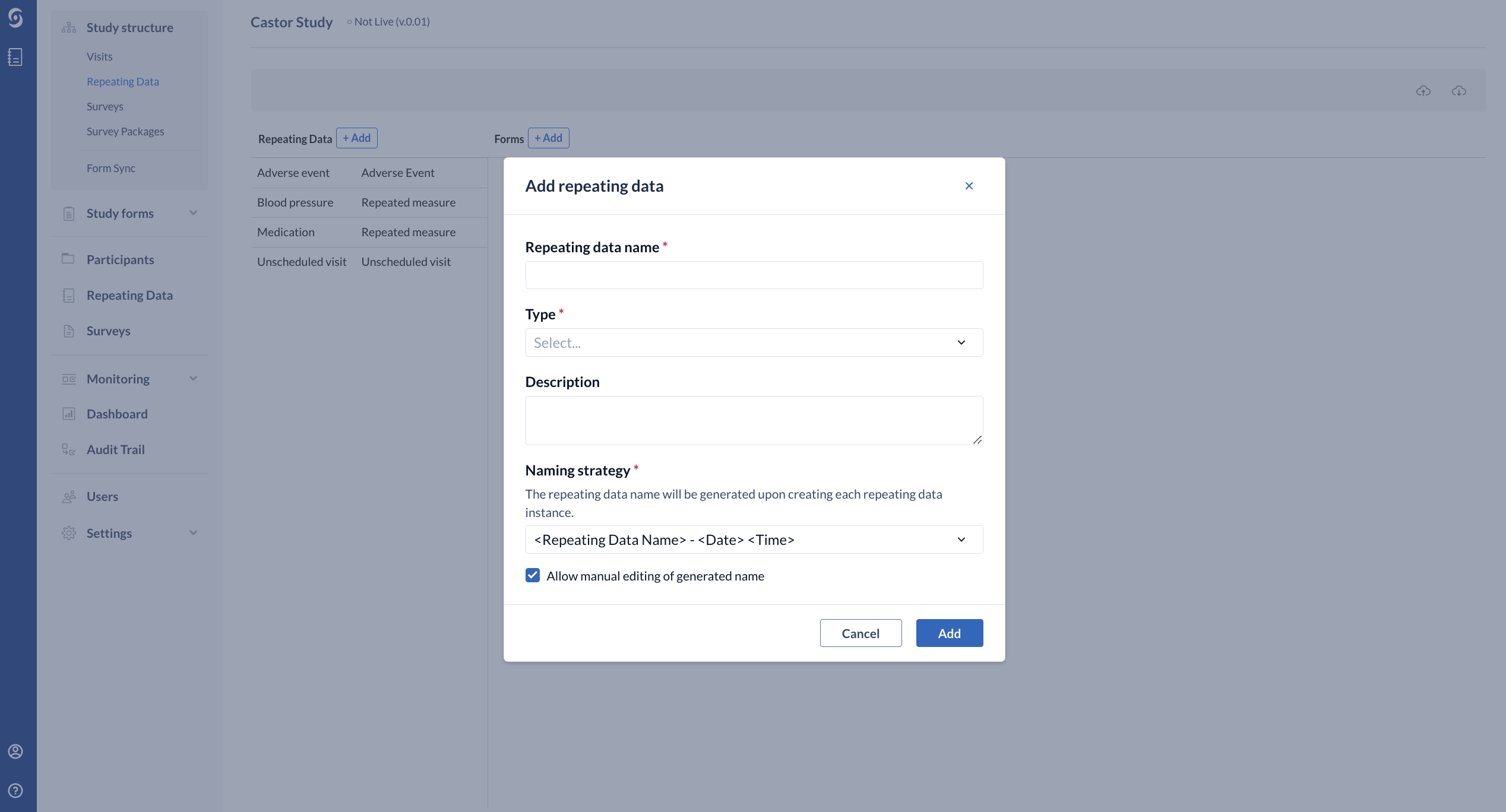
- Required - enter the Repeating Data name
- Required - select the relevant Repeating Data Type from the dropdown menu - Adverse Event, Event, Medication, Other, Repeated Measure and Unscheduled visit. The Repeating Data type "repeated measure" may only consist of one form.
- Optional - you can provide a short Description of the Repeating Data structure, if preferred.
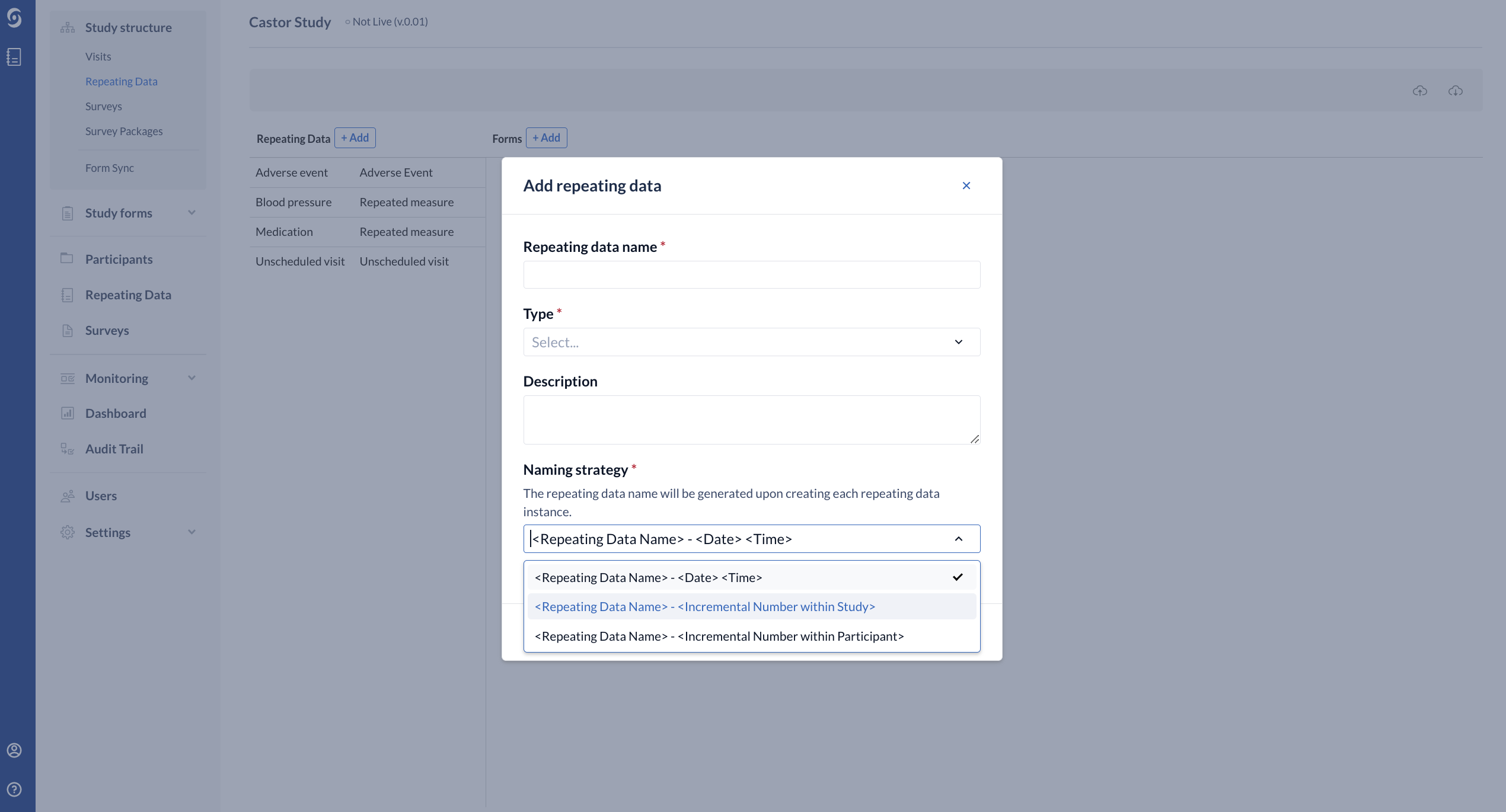
- Required - Repeating Data Naming Strategy - allows you to specify how Repeating Data will be named when an instance is created.
- By default this is set to <Repeating Data Name> - <Date><Time> (for example, Adverse Event - 22-04-2021 13:12:11).
- It is also possible to choose generate Repeating Data based on the incremental number within study <Repeating Data Name> - <Incremental Number within Study> or within a participant <Repeating Data Name> - <Incremental Number within participant>.
- Allow manual editing of generated name - possibility to allow edit the Repeating Data name during the data entry.
Once the fields are complete, click 'Create Repeating Data' to return to the editor. The new Repeating Data structure will be displayed in the sidebar. To add forms to your Repeating Data, click the '+ Add' button to create a new form in the Repeating Data:
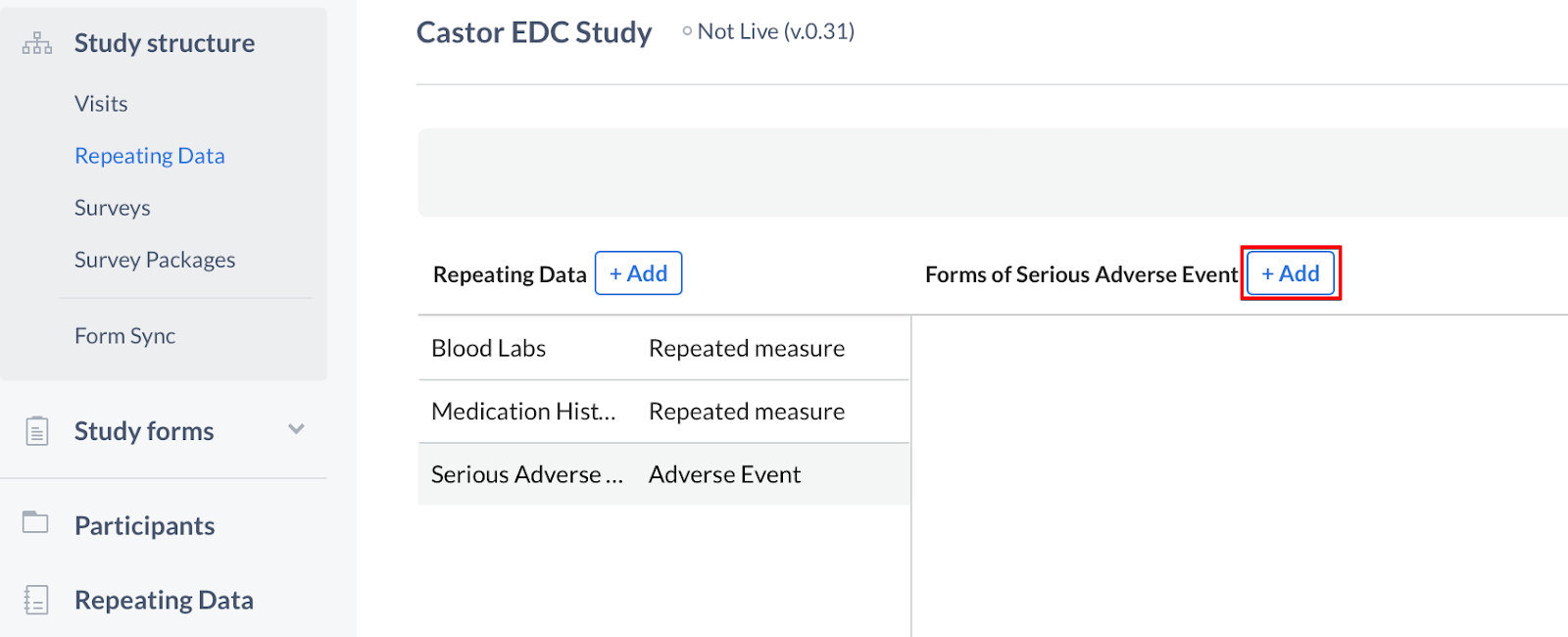
Add a form to the Repeating Data by specifying its position, name and description - click 'Save' to save the form. The Repeated Measure type can only have one form.
Add fields to the Repeating Data
When finished adding forms, navigate to the 'Study forms' tab to start adding the fields to your Repeating Data forms:
- Select the 'Repeating Data' sub-tab
- Choose the Repeating Data you created from the Repeating Data dropdown
- Select a form to which you want to add fields
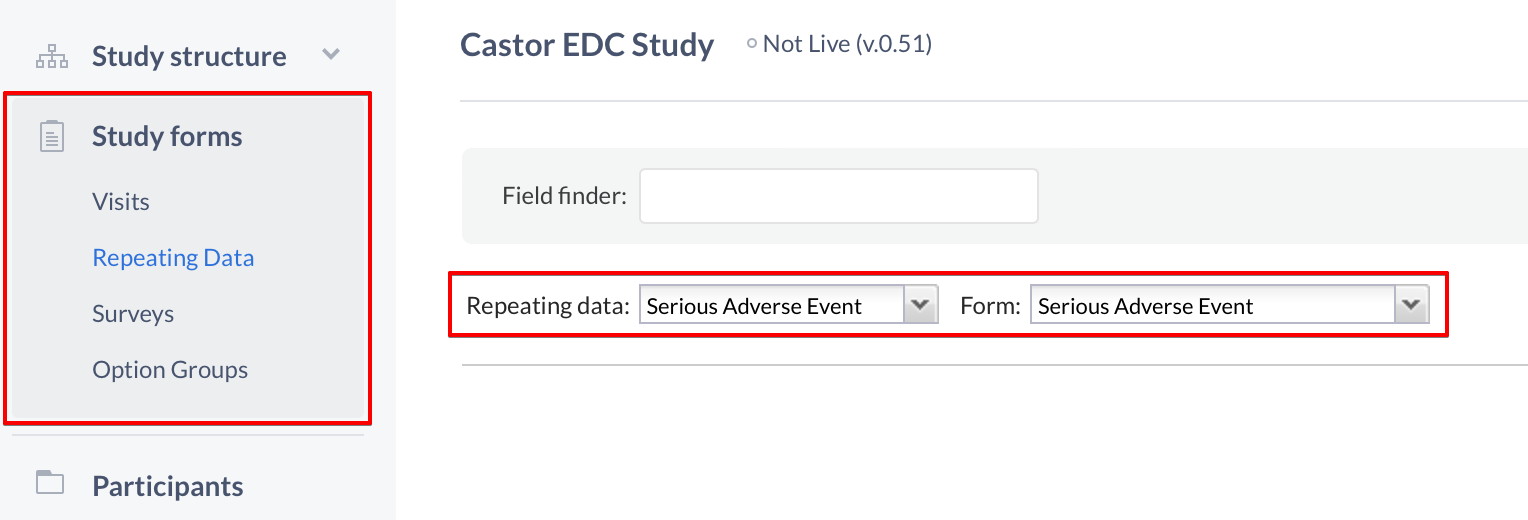
You can now start adding fields to your new Repeating Data, and you can try making a Repeating Data button to enable quick access from your CRF to the Repeating Data.
Edit a Repeating Data or Repeating Data form
Once you have your Repeating Data or Repeating Data form(s) created you may need to make changes to any of the fields that you needed to fill out when you created them. Some examples are the name and the position of the form, or the Repeating Data type.
To make changes to these fields, once again you need to use the cogwheel close to the name of the Repeating Data or Repeating Data form. This time you will select the option "Edit Repeating Data" or "Edit form":
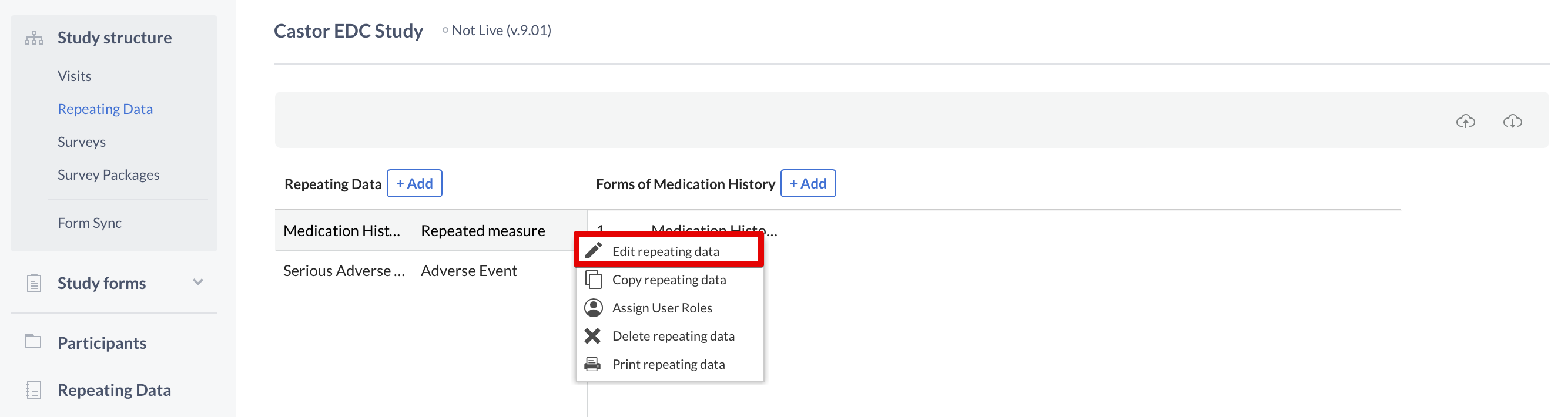
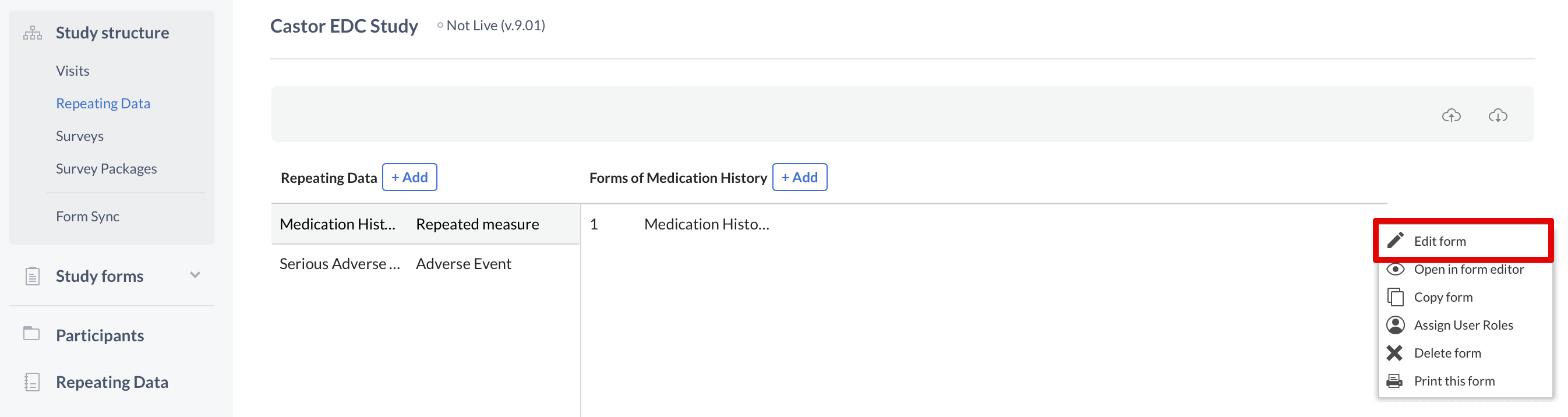
Introduce your changes and click "Save" when you are done.
Delete a Repeating Data or Repeating Data form
To delete a Repeating Data or Repeating Data form, click on the cogwheel next to the concerning Repeating Data or form and select the option "Delete Repeating Data" or "Delete form":
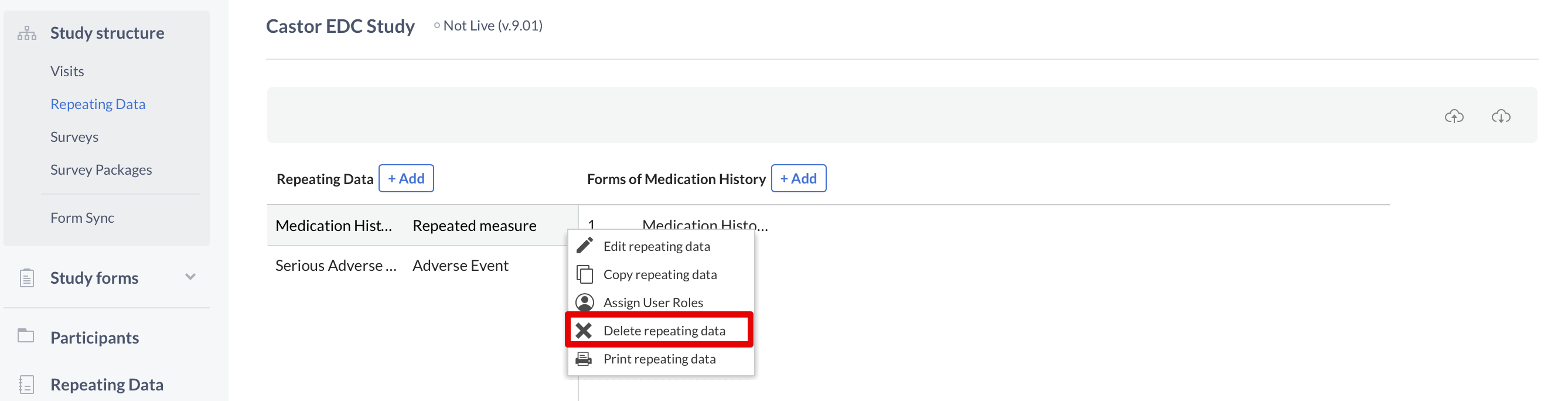
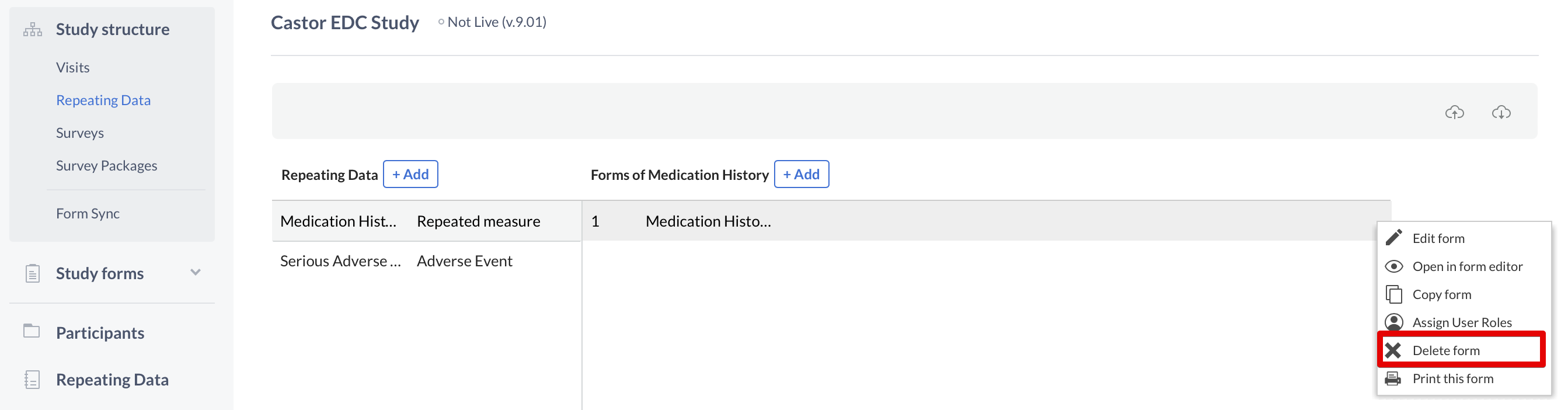
Next, enter the exact name of the Repeating Data or Repeating Data form in the confirmation pop-up that appears. Pay attention to the upper- and lower-case letters of the name:
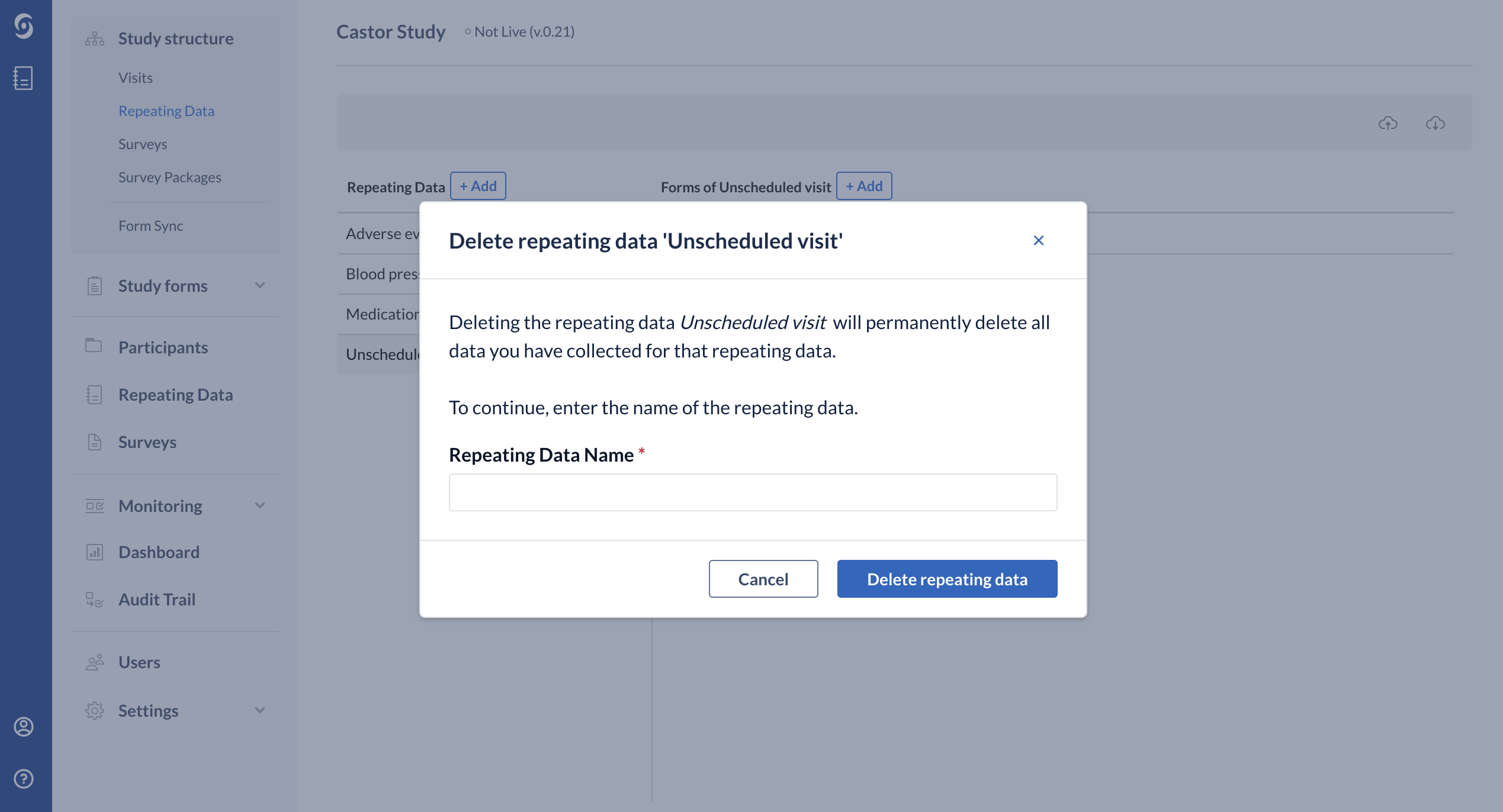
Click "Delete repeating data" and now the structure is no longer visible in the list.
In case there are existing repeating data instances, it will not be possible to delete a repeating data structure and an error message will be shown:
You cannot delete this repeating data yet. There are 1 repeating data instance(s) created for this repeating data. You must first delete all instances. Note: Deleting instances is only possible for studies that have never been set to live.
If the study has never been set to live, you can delete the repeating data instances first, then delete the repeating data structure. If the study has been live, it will no longer be possible to delete the repeating data instances. You can hide it from all user roles by assigning roles to the users and hiding this particular form using the ‘Assign User Roles’ feature.