Hide parts of the CRF for certain users in CDMS
You can hide parts of the eCRF for certain data-entry users. This means that they will not see a specific part of the form while doing data entry.
All users with 'Manage forms' rights will still have access to the full form in the ‘Study Structure’ Editor
You will first need to define user roles as per this article.
To hide parts of the eCRF for a specific role, follow these steps:
- Go to the Study Design > Study Structure
- Find the section of the study that you want to hide
- From the three dot menu, select the option to “Assign user roles”
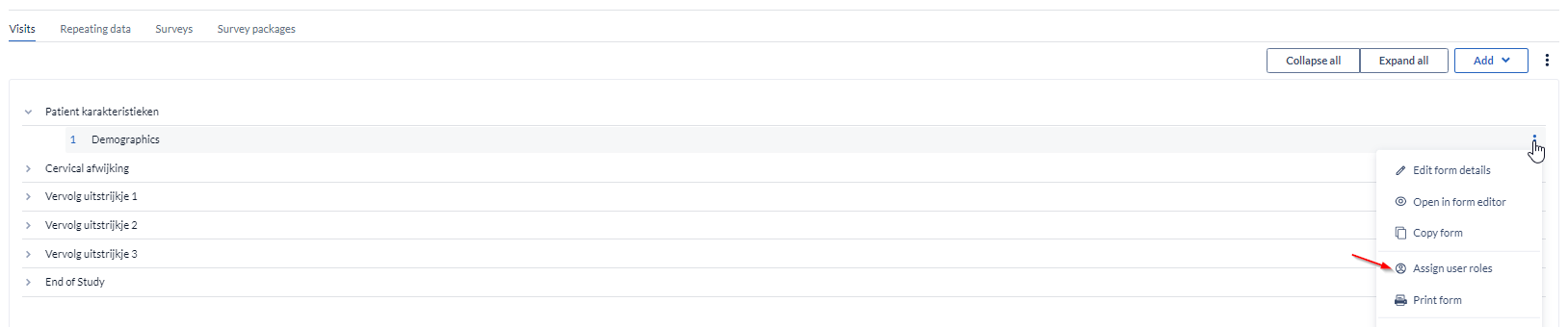
- Select the roles which won't have the right to view the visit/form
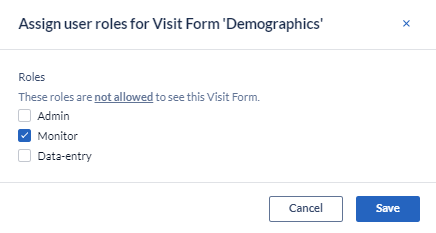
- Save the change
You can choose to hide a study visit, a form, a repeating data or a repeating data form.
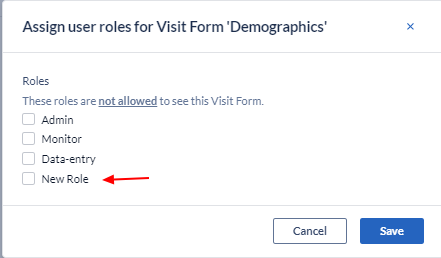
As the study progresses, and you add new roles, these will also be displayed as a selecting option: