The 'Repeated measure' field in CDMS
Table of Contents
Repeated measurements are a small set of related data points that semantically belong together and that are measured from 0 to N times. A good example is repetitive measurement of blood pressure, which can consist of several related data points that are always measured/registered together.
For example, you could use a repeated measure field if you want to repetitively register the following elements:
- Blood pressure (systolic and diastolic)
- Position of the patient during measurement
- Date and time of measurement
Even though it is called a 'measurement', the repeated measure field can also be used for registering repetitive events that are not necessarily 'measurements'. For example, if you want to repetitively register medications during your study, the repeated measure field can be used to register the medications during each visit.
Report type: repeated measure
Before a repeated measure field can be added to the study form, a repeating data form type called repeated measure should be created. For more information on creating a new repeating data form, refer to creating reports.
Creating a repeated measure field
Once you have created the repeated measure form, you can add a repeated measure field to the study.
To create a repeated measure field, go to the Form builder and navigate to the appropriate study visit and form and select the field type 'Repeated measure'. You can also add this field within another repeating data form. Define the properties of the field:
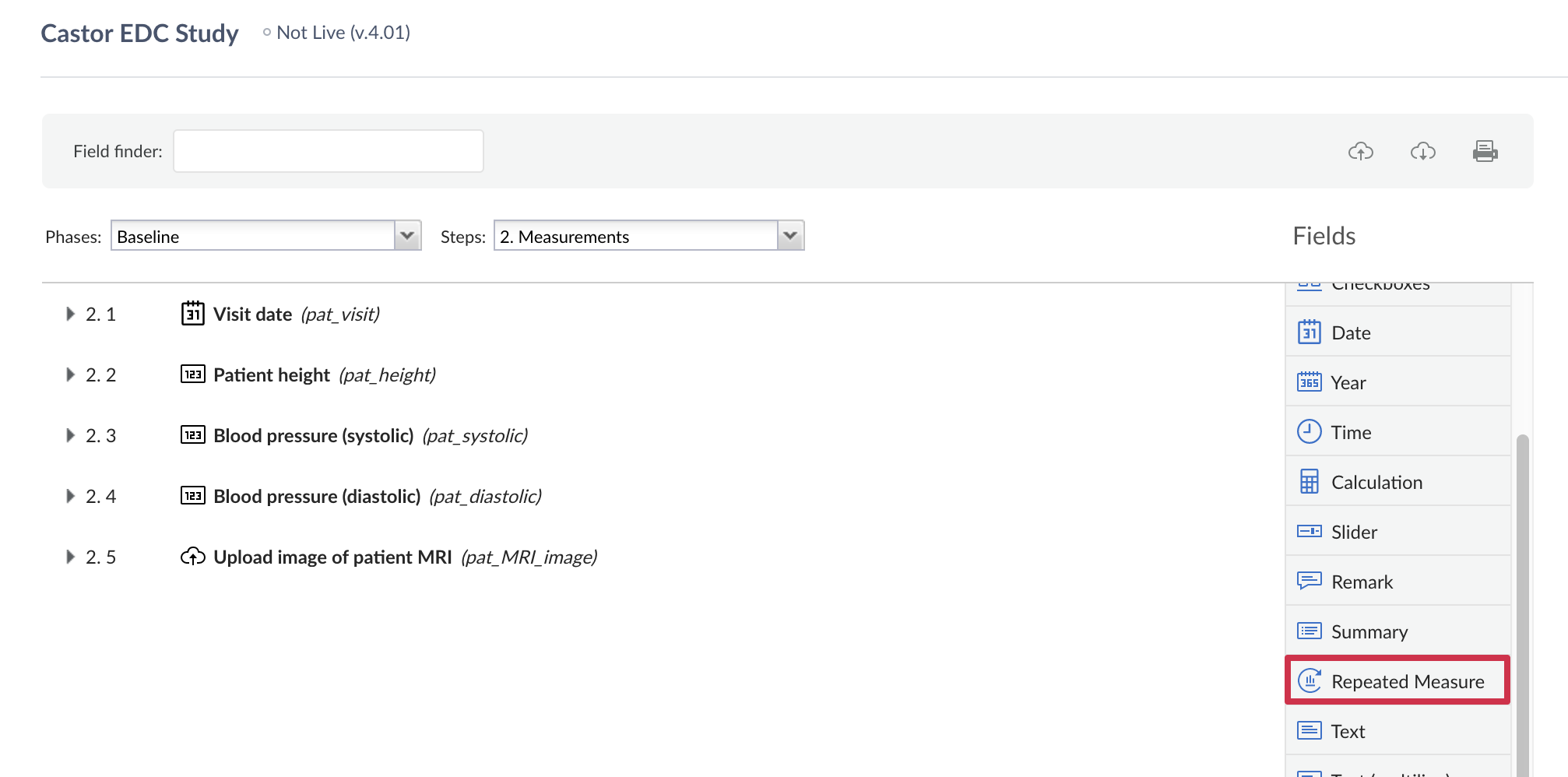
When creating a repeated measure field, you can choose to display measurements for all linked visits or repeating data form for a specific repeated measure:
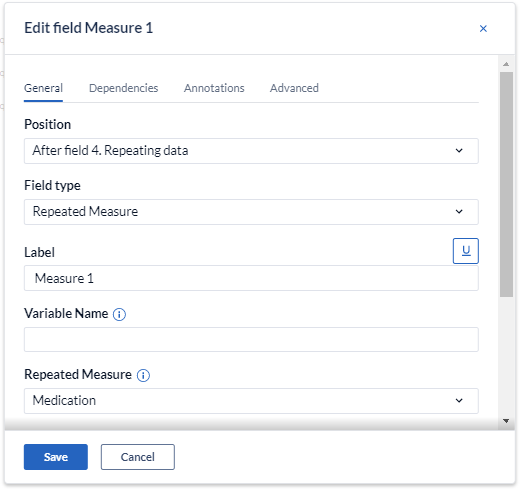
You can also edit the ‘’Button label'' that will appear in data entry.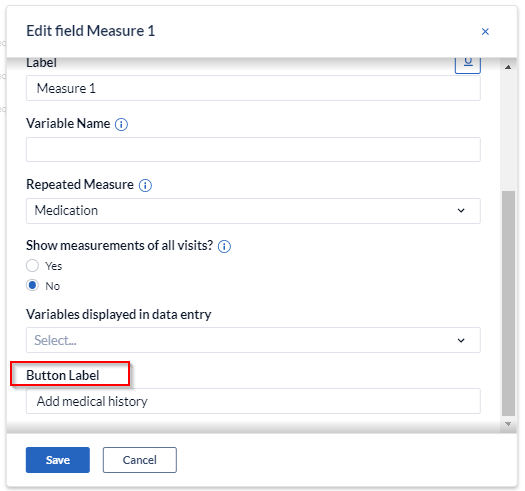
It is also possible to create calculations within repeated measure fields. For example, a score can be calculated as part of each measurement. However, it is not yet possible to calculate the number of measurements in a calculation field.
In data entry, the repeated measure field looks like this:
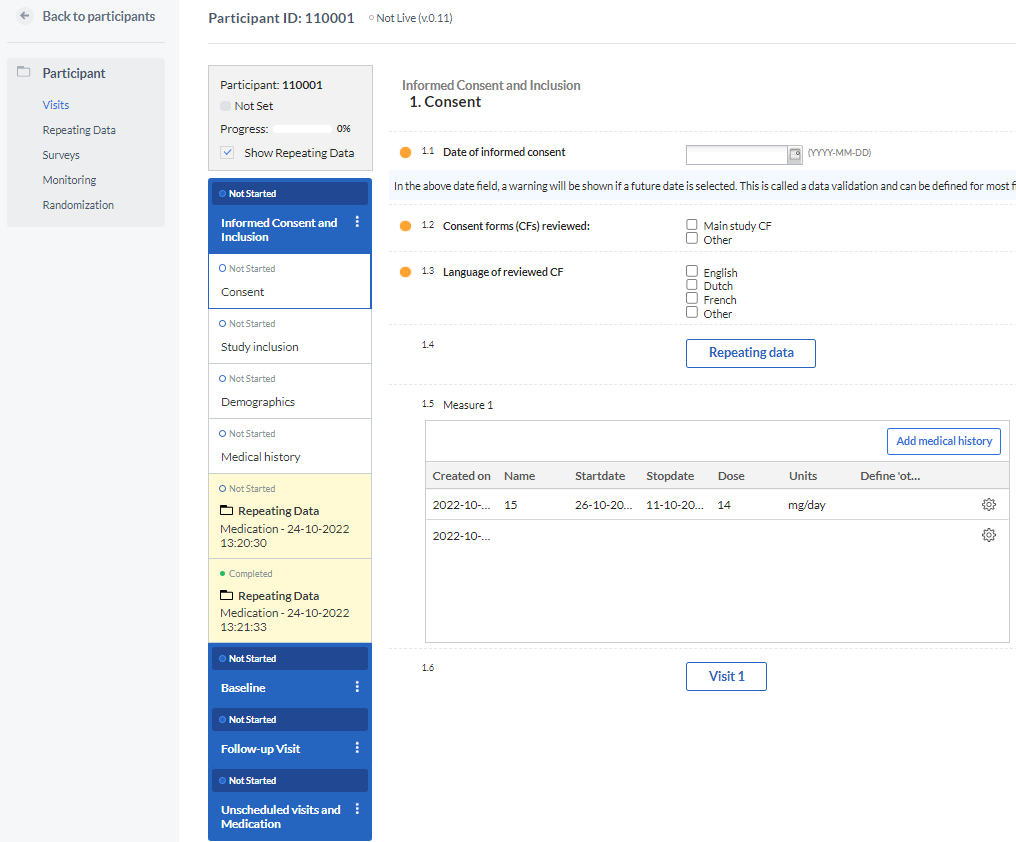
By selecting 'Add medical history' (or the label of your choice), measurements can be easily added within the form and will be shown in the form of a grid, representing each measurement as a new row and each field that is part of the repeated measure as a separate column.
Each measurement will be created in the form of a new repeating data instance and will appear in the Repeating data tab. Each repeating data form will be attached to the visit (or repeating data form itself) where the repeated measures field is located.
Importing repeated measure data
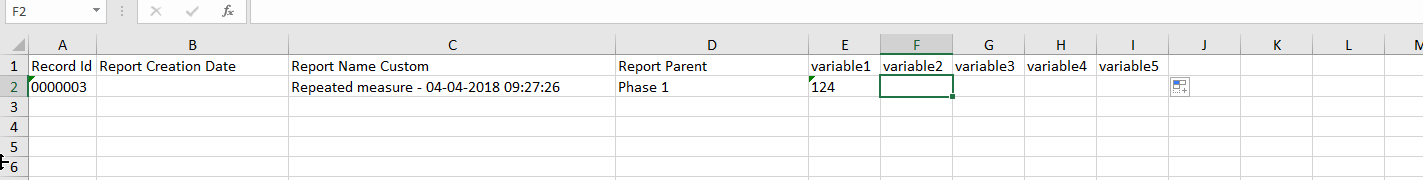
First, export the repeated measure to Excel. Even where no data has been entered to the repeated measure, the exported spreadsheet will provide a template containing the columns that need to be completed and data can simply be added to the corresponding columns. The 'creation date' column should be left blank, as this is automatically created with the creation of the repeating data form. This is what a prepared spreadsheet for import should look like:
Be aware that, unlike the study import method, you cannot create new participants while importing repeated measure data. The participants must exist in Castor before the data can be imported.
To import the repeated measure follow the instructions on the page Import repeating data.