The 'Summary' field in CDMS
Table of Contents
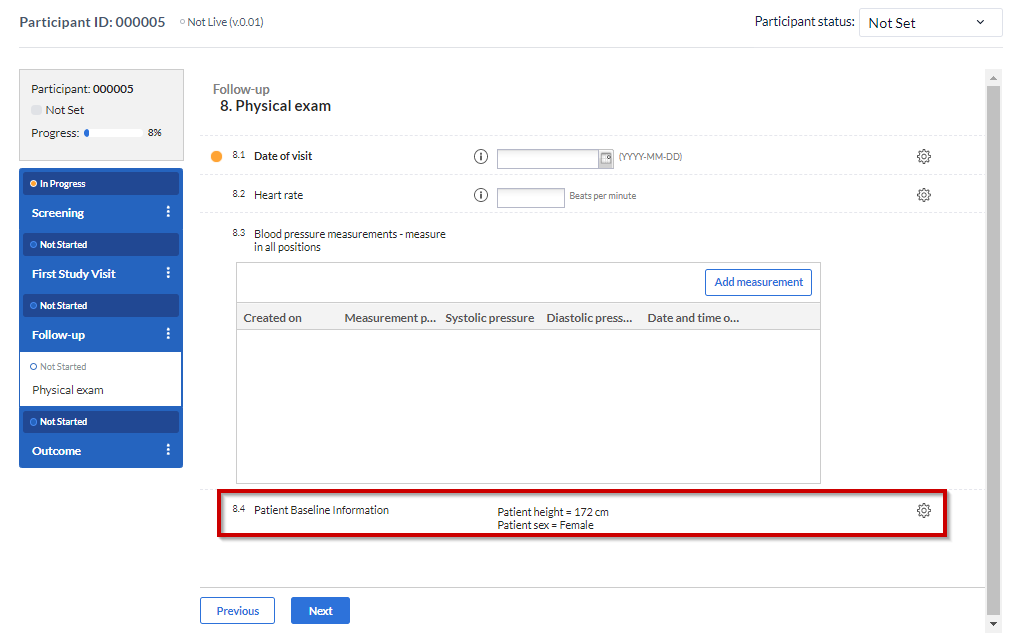
The summary field is used to provide the user with summaries of entered data during data entry. This can be useful to remind the user of information which was entered during earlier forms or visits in the study.
The summary field is used by specifying a template that references other fields for data. This is done by using the field's 'Variable name'. Before creating your summary field, please enter and note the 'Variable name' for the fields that are to be used in the summary e.g. gender (dem_sex).
Note:
- Summary fields are not included in the data export.
- Avoid making fields dependent on the summary fields, as this will not trigger a dependency, since the values in the summary fields are not saved and only displayed in the user interface.
Create a summary field
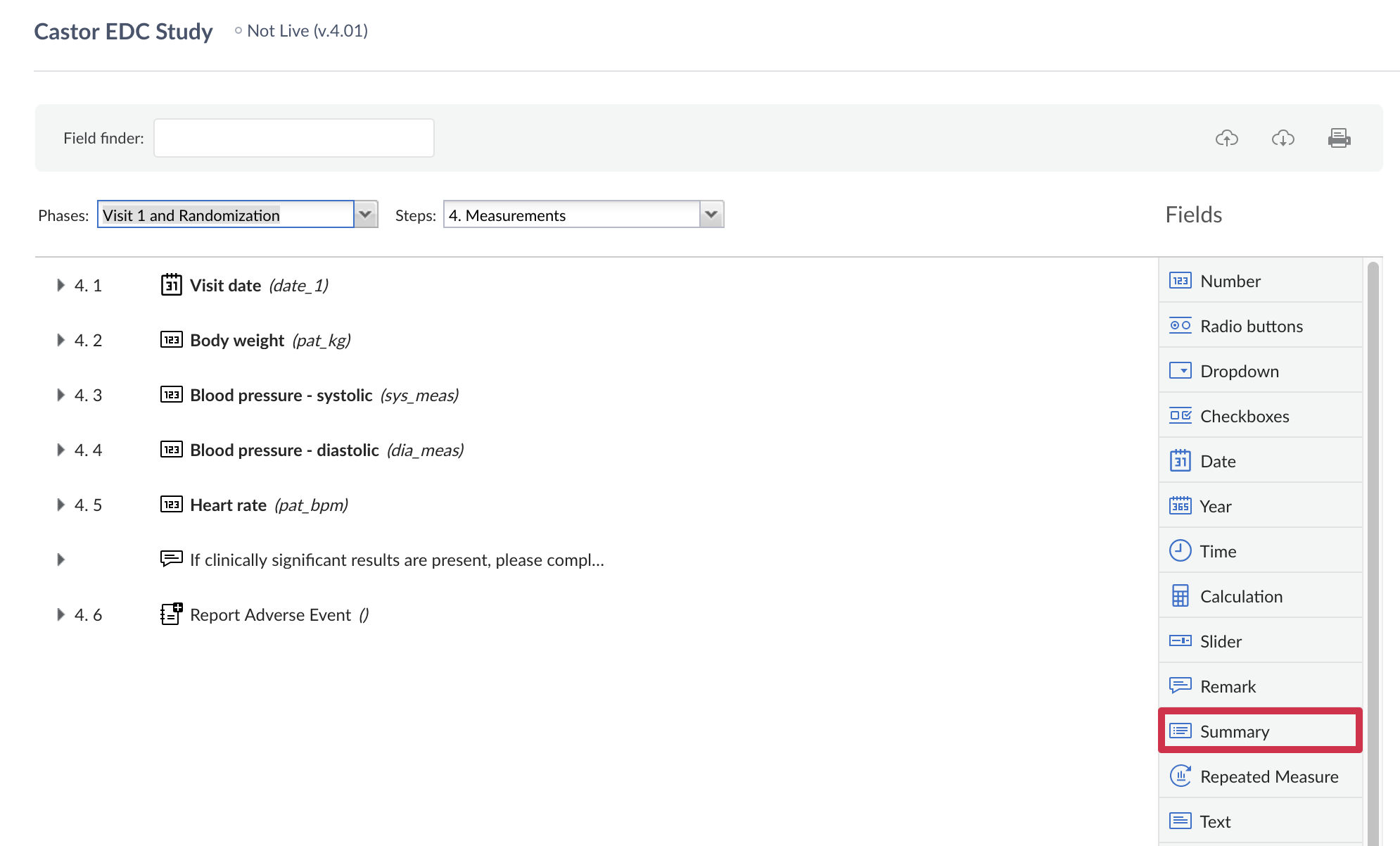
1. Create a summary field by clicking the 'Summary' button in the list of field options:
2. In the 'Add a new field' dialog window, provide the field label and create the summary template as can be seen in the example below:
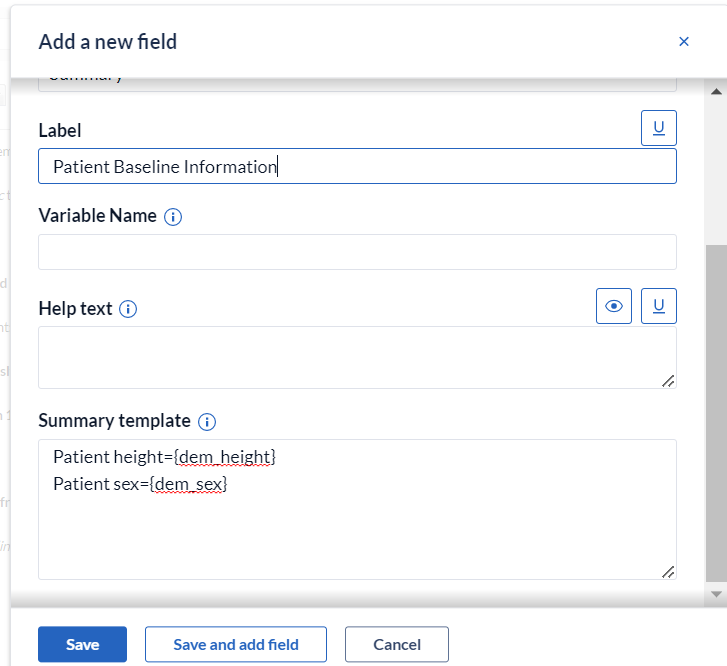
3. To reference fields, braces '{}' are added around the field's variable name. For instance, in the above example patient gender is referenced by entering {dem_sex}.
When a participant is created and filled out, the summary field will appear as seen below: