Castor eConsent Release Notes 2022.4.x.x
Table of Contents
Major release 2022.4.0.0
Release dates:
EU server: October 26, 6 pm CEST / 12 pm EDT
US server: October 27, 10 am CEST / 4 am EDT
New features and enhancements
Print-to-sign workflow for paper signatures
The management of paper signatures, which was made available in the previous major eConsent release, is now enhanced by supporting a full print-to-sign workflow.
The ICF builder for ICF templates that use a paper consent method now allows pdf file uploads. This means that the finalized “paper” ICF file can be uploaded against the eConsent paper ICF template. By doing so, the actual content of the ICF becomes available for site members. The site member can download and print the ICF template directly from the eConsent subject record, in order to make sure the right ICF version is getting signed. Once the printed version is signed by all parties, a scan can be uploaded into the system to ensure proper tracking, together with the electronically signed ICFs.
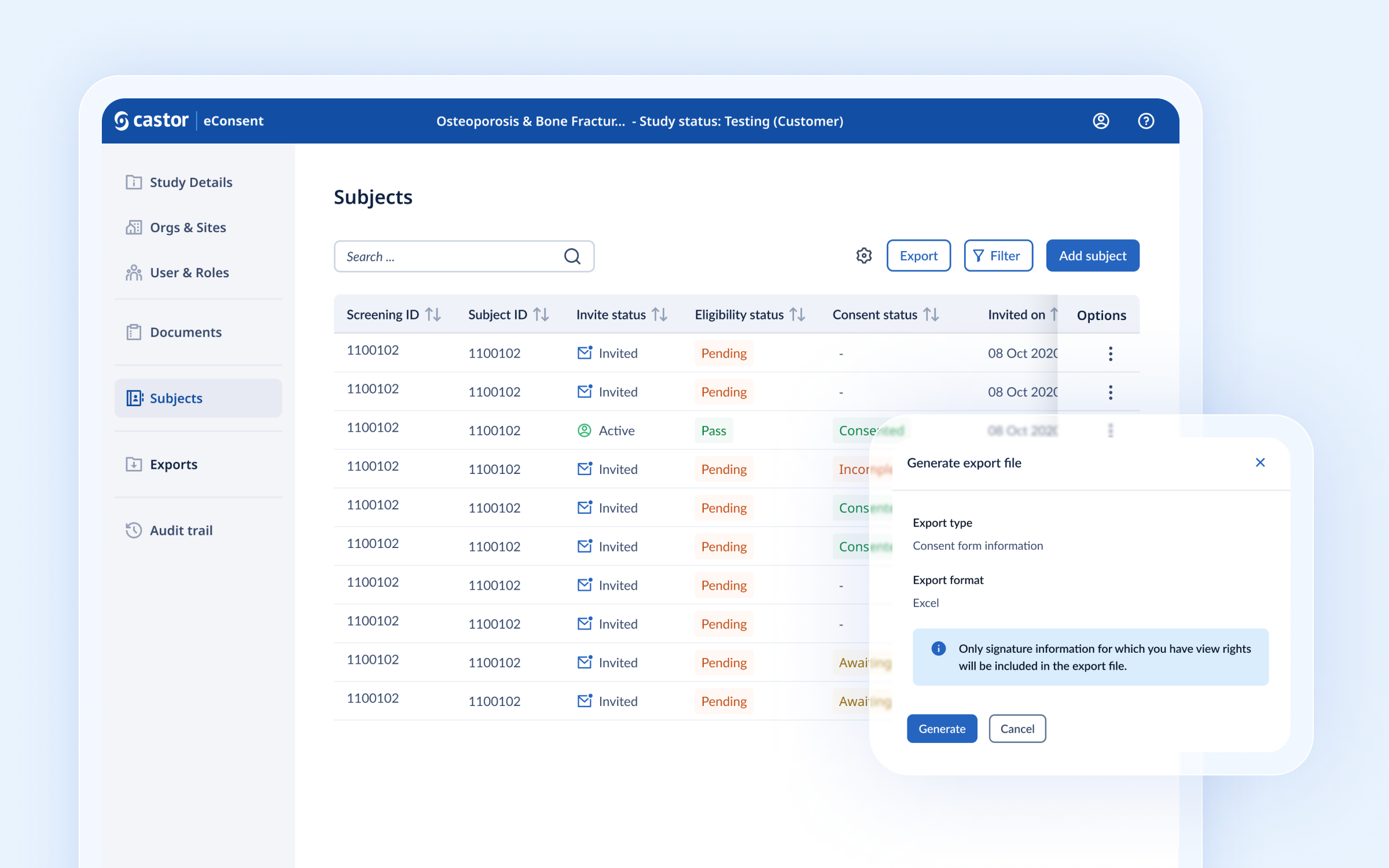
Exporting
The export report which contains data on the informed consent and signature status is now available for users to generate directly from eConsent.
How to export
Users with the Study Investigator or Study Monitor role are able to generate the export that contains signature information. Exporting is done via the ‘Export’ button in the general Subjects overview. Once the export is generated, the requester is notified by an email that includes a personal download link. The generated export can then be downloaded from within eConsent.
Searching and filtering on the Users & Roles overview
The Users & Roles overview is enriched with new capabilities to search and find the right data. eConsent users can now search on user email address and filter the view based on user role and site.
Webhooks
More efficient integrations, between eConsent and external systems, can now be supported by leveraging the eConsent webhooks mechanism. The available webhooks cover all the subject/ICF related events. For example, given that there is a subscription on the event of signing an ICF by a subject within a study, when a subject signs an ICF, then eConsent sends an API call with that event to the external system. Documentation on how to implement webhooks is available upon request.
Other enhancements
Subjects are now able to re-sign the same ICF version they already signed. This could be used to correct for mistakes that are surfaced after the ICF is signed. All the signed ICFs are maintained.
The studies in the study overview are now sorted from most recently created to oldest created.
Enhancements to the Castor CDMS linkage: eConsent can trigger participant record creation in a linked Castor CDMS study when the eConsent record is set to eligible. And it is now possible to configure screening surveys to not be sent out automatically when a subject is added to the study, but require a manual send action instead.
Enhancements to the Single Sign-On (SSO) capabilities: Users with a hybrid account (having both a regular eConsent account and a SSO account with the same email address) will be forced to use the SSO account when opening a study and signing an ICF that is configured to have SSO. A user with a hybrid account that is logged in with their eConsent account can see all their related studies in the overview, however, they are not able to open studies that require a SSO login.
The column ‘Additional signature(s)’ is added in the Consent forms table within a subject record. This column displays the signature status for additional signatures, like a LAR or second study team member signature.
The wording in the dialog to confirm the signers’ identification when starting the in-person signing flow is improved.
The wording of the 'Consent method' option group when adding an ICF to a subject is improved.
System defects fixes
Here’s a list of the most important fixes included in this release:
Fixed the sorting behavior of the Org & Sites menu, as it used to work per page instead of for the complete list of sites.
Fixed an issue where the 'Consented on' date was not removed when removing the uploaded paper signed ICF.
It is no longer possible to remove a site from a published ICF template if that ICF template is in use by a subject from that site.
The date on the ICF was always displaying in English, instead of displaying in the ICF language.
The PDF download of an electronic ICF now only contains a header for Legally Authorized Representative (LAR) agreements when the form actually contains agreements.
Fixed an issue where for a specific scenario it was not possible to update a user role.
Fixed an issue where directly after a release the new added labels were only visible after a hard-refresh.
Fixed an issue where the pending study invitations were in the same list as the already accepted study invitations.
Fixed an issue where an incorrect list of study team members was showing during activating a second optional study team member signature.
Fixed an issue where the ICF invite email to the subject was not sent to the updated email address.
Fixed an issue where it was not possible to send an email notification to the second study team member signatory in the in-person signing flow.
Fixed an issue where the 'Site(s)' field in the 'Manage user' modal was not displaying the assigned sites.
Fixed some styling issues in the "Personal details" card within the Subject profile.
Fixed some styling issues on the screen on which the participant can fill in their personal details.
Hotfix release 2022.4.0.1
Release date EU and US server: November 1, 12 pm CET, 7 am EDT
System defect fixes:
- Fixed an issue where it was not possible to submit a signature on an ICF for subject records that were created via the API instead via the user interface.
Maintenance release 2022.4.1.0
Release date EU and US server: November 4, 11 am CET, 6 am EDT
Enhancements:
- User feedback tool GetFeedback is implemented so users can send feedback about eConsent to Castor directly through the platform.
System defect fixes:
- Fixed an issue where for some specific users it was not possible to invite other users (subjects and study team members) to their study.
- The signature date on the ICF was displaying in English on the web version, instead of displaying in the ICF language.
- Removing the paper ICF file from a duplicated ICF template will now no longer remove the file from the original paper ICF template as well.