Castor eConsent Release Notes 2023.3.x.x
Table of Contents
Major Release eConsent 2023.3.0.0
Release dates
EU server: December 13, 6 pm CET / 12 pm ET
US server: December 14, 9 am CET / 3 am ET
New features and enhancements
- A PII Removal process can be run upon request. This process removes any PII in the participant details for a selection of participants in a study.
- When the PII Removal process is initiated, a new audit trail event will be logged for each participant of which the PII is removed.
- When the PII Removal process is initiated, then PII in historical ATEs is also removed.
- When the PII Removal process is initiated, then the participants of which the PII will be removed are also archived within the study.
- When PII of participants has been removed, then this will also be reflected in exports.
- After the PII removal process has run, the participant details of a record can still be changed afterwards.
- After the PII Removal process has been run, then this will be reflected in exports. For example, if a participant's first name is changed to 'PII Removed', then this will also be shown in an export.
- It is possible to retrieve an export of all participant details through the UI from the participant list.
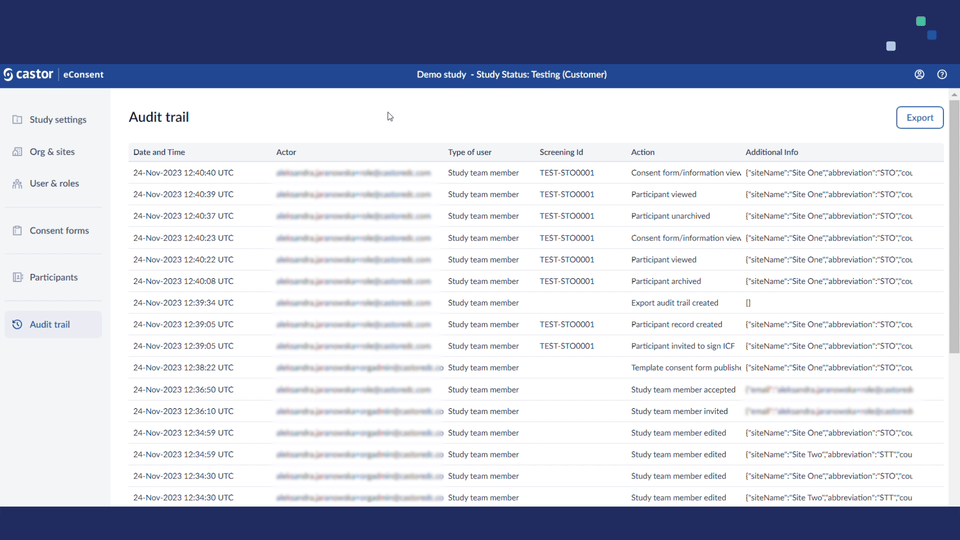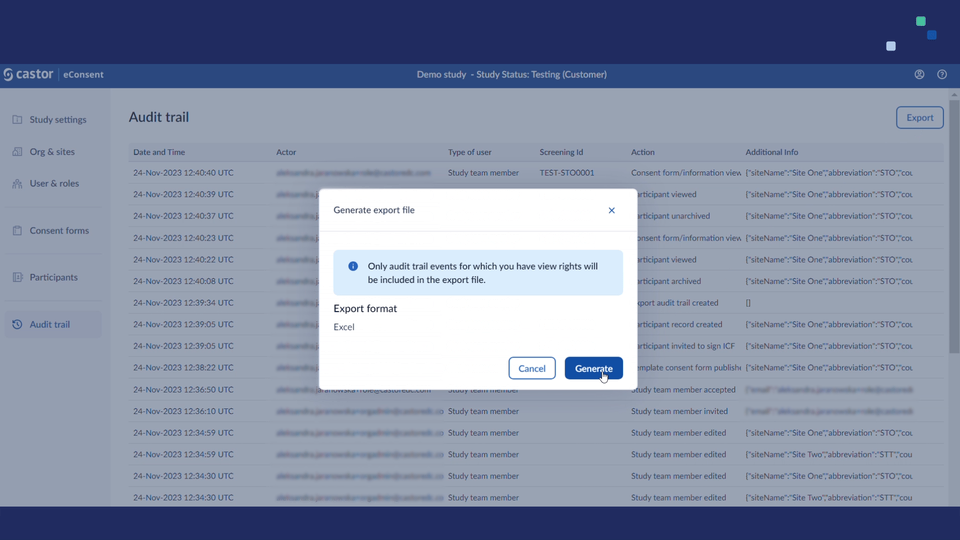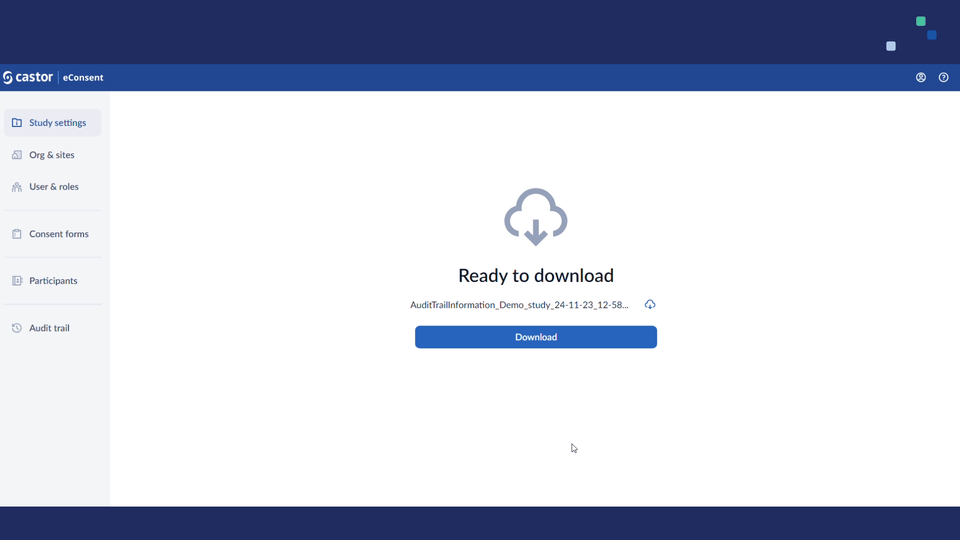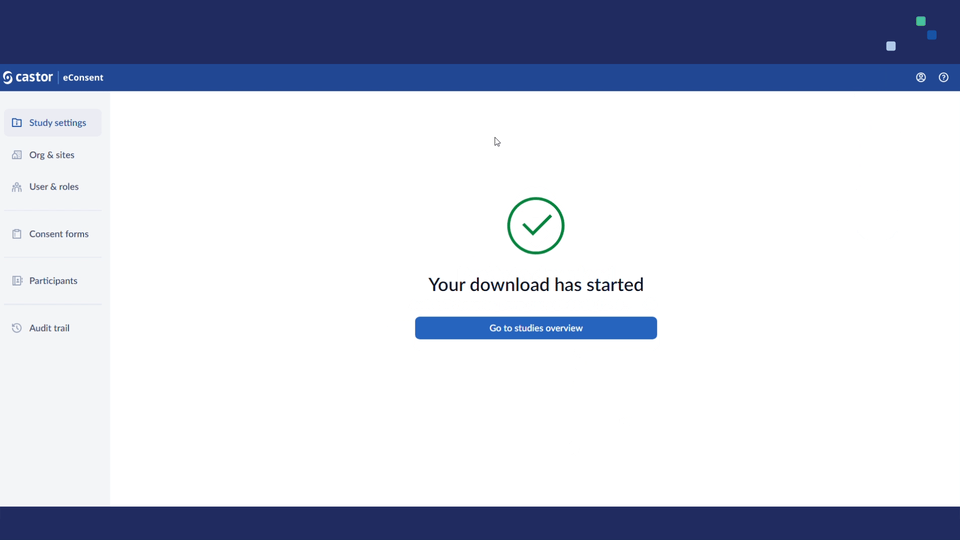
- Added support for exporting audit trails. Study roles with PII access (i.e. study investigators/monitors) will see an 'Export' button on the audit trail page they can use to run the export.
- Minor revision in the wording of the invitation email for LAR users.
- When generating a PDF out of your ICF, the ICF version and its approved date were already displayed. We are now also including, when available, two new optional pieces of information: Protocol Version & IRB Approval Date.
- The setting 'Lock ICF by default' implied that you can not lock the ICF after locking again, which is not correct. Therefore the setting was renamed to 'Lock-unlock signing process'.
- The answer option limit of participant questions has been increased from 5 to 20.
- Castor can initiate a process where PII is removed from specific participants within a study. This can be done based on a .csv file with a list of Screening IDs for which the PII needs to be removed.
- After archiving a participant, it is not possible to change the participant details anymore. After unarchiving this will be possible again.
- Instead of showing a general error, the user will now be properly informed that they can't delete a site that is integrated with CDMS.
- Castor Admins can now remove integrations instead of having to rely on Castor Engineering to do this for them.
- Support for subscribing to a webhook for paper ICFs, so you can be informed whenever a paper ICF is uploaded.
- Improved the 'Locked ICF' indication on mobile devices to make it clearer and easier to understand for the participant.
-
Added a link to the Castor Academy in the Support menu.
-
The following API endpoints are removed and can not be used anymore:
- informed_consent_form_countries
- informed_consent_form_study_sites
- study_roles
- study_sites_roles
- subject_roles
-
subject_appointments
- Adjusted the order of the settings in the 'Configurations' tab of the study settings to reflect the steps in the participant journey.
System defect fixes
- Fixed an issue where it was not possible to remove a study role from a study team member that also had specific site roles.
- Fixed an issue where adding an ICF to a newly added participant could lead to an error. This would only happen in studies with a CDMS integration and right after you deleted another participant in CDMS and archived it in eConsent.
- Fixed an issue that was throwing an error message when user was refreshing the page while adding a participant or an ICF.
- Fixed an issue where eConsent would throw an error message when a user would refresh the page while adding a participant or an ICF.
- Fixed an issue where it was possible to add new ICFs to archived participants.
- Fixed an issue where the Screening ID of archived participants would not be preceded by 'ARCHIVED' in exports.
- Changed the wording of 'Subject' to 'Participant' in the ICF Information exports.
- Changed the wording of 'Subject' to 'Participant' in the Participant Detail exports.
- Fixed an issue where the 'Old value' wouldn't be shown in an audit trail export.
- Fixed an issue where an extra bullet point would show on the pdf version of an ICF in case of a single, indented bullet point.
- Fixed an issue where unarchived participants were not able to sign a document anymore.