Add a comment to a field in CDMS
Table of Contents
Adding comments to a field
A comment is a note that users can add to a field for additional context or information. Users with "View" permissions can check and enter comments to fields using the dedicated action.
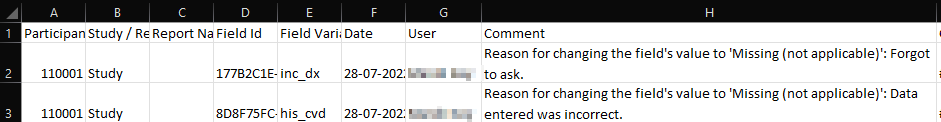
- You can add multiple comments, but it's important to know that comments cannot be deleted or edited once added.
- When exporting study data in CSV or Excel formats, comments are included in a separate field.
- Note: Comments are not included when exporting using the SPSS option.
Example of Comments Export in Excel/CSV Format:
Modern Data Entry (from 2024.2 release)
- Click the context menu (three dots) next to a field.
- Choose the ‘Comments’ option.

- Enter your note in the Comment section.
- Click the ‘Add Comment’ button to save it.

Data Entry View (Versions Prior to 2024.2)
- Open the menu for the field by clicking the cogwheel on the right side of the field.
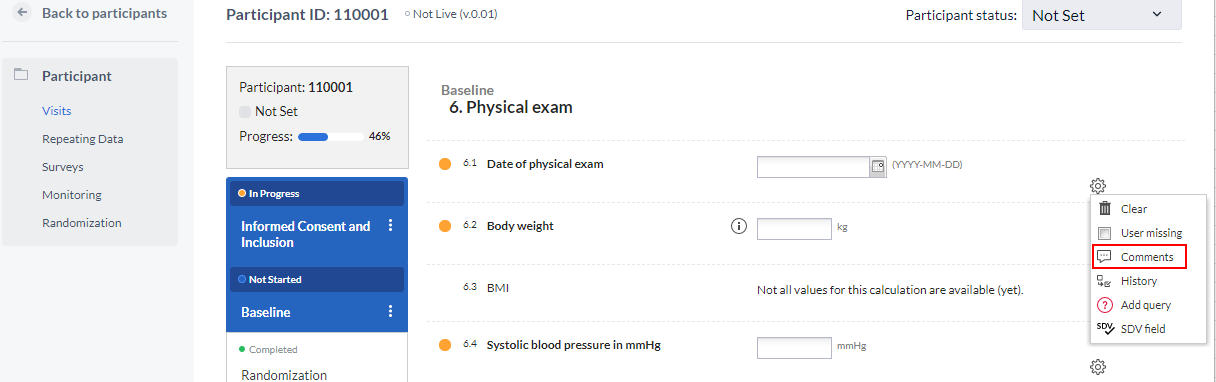
- A dialog will appear where you can enter your comment. Click Add comment button to save the changes.
