The 'Link' field in CDMS
A ‘Link’ field allows to create a link to an external webpage that is generated in the same way as calculation and summary fields are generated, using variables. With this approach you can create deep-links to external resources like PACS, HIS or other systems.
Using a custom link requires a link template. The active link will be created automatically. To create a 'Link' field in your forms, navigate to the Form builder and click on the Link field.
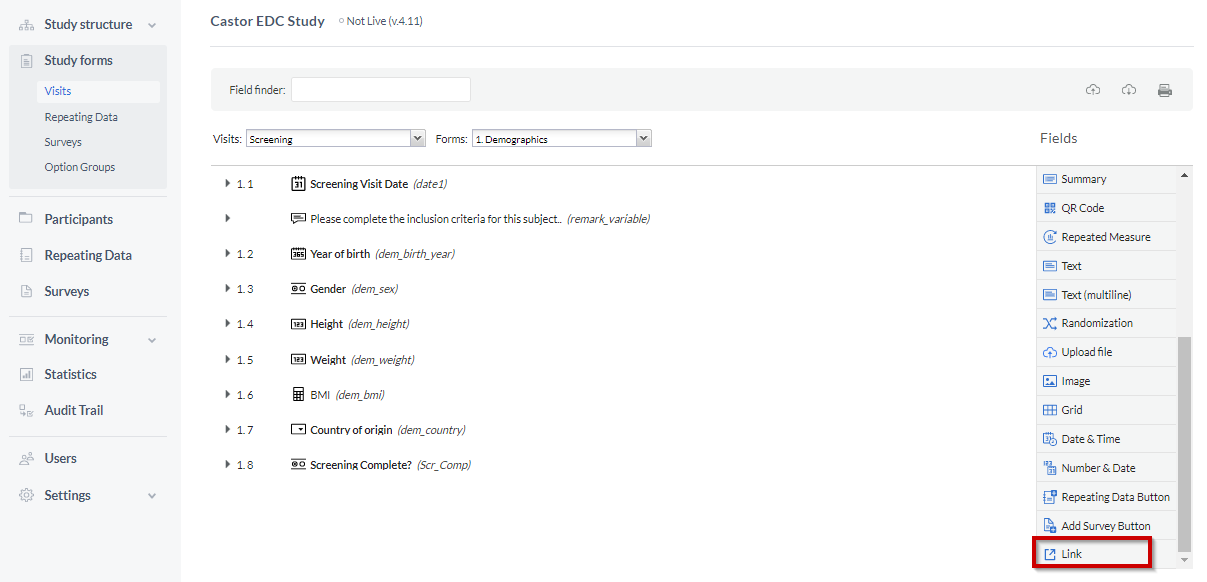
In the Link template field, add a link and a variable name:
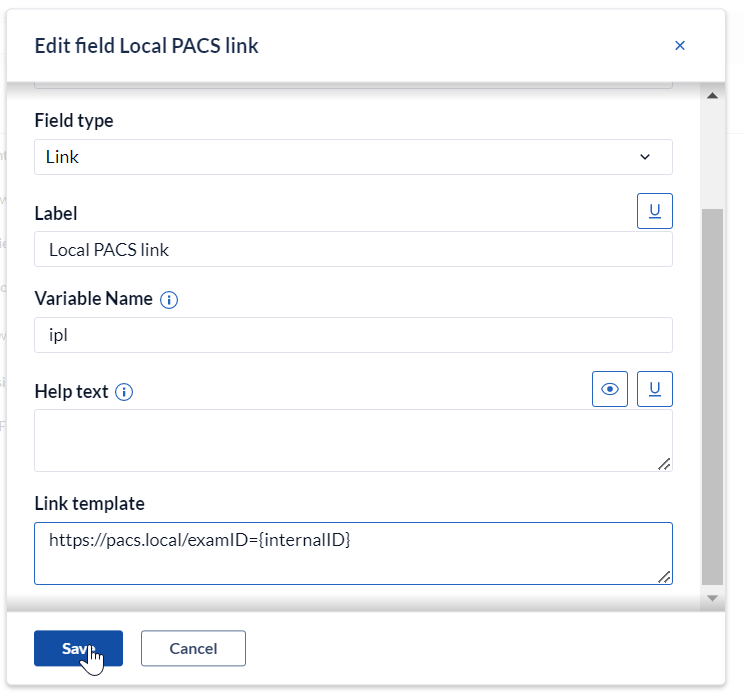
Along with the variables defined in your study, it is also possible to use predefined variables such as "{recordId}", "{userId}".
When opening a participant in the data entry view, the link field will appear as follows:
.png)
As an additional safeguard the warning message will appear informing the user about leaving the Castor EDC/CDMS.
.png)