How do I set my study to live in EDC/CDMS?
To set your study to live, you need the 'Manage Settings' right.
Before you can set the study to live, you must complete the sections 'Study properties' (2) and 'Billing Information' (3) in the study's 'Settings' tab and ensure that you have selected a user in the 'main contact' dropdown in the 'General' (1) tab.
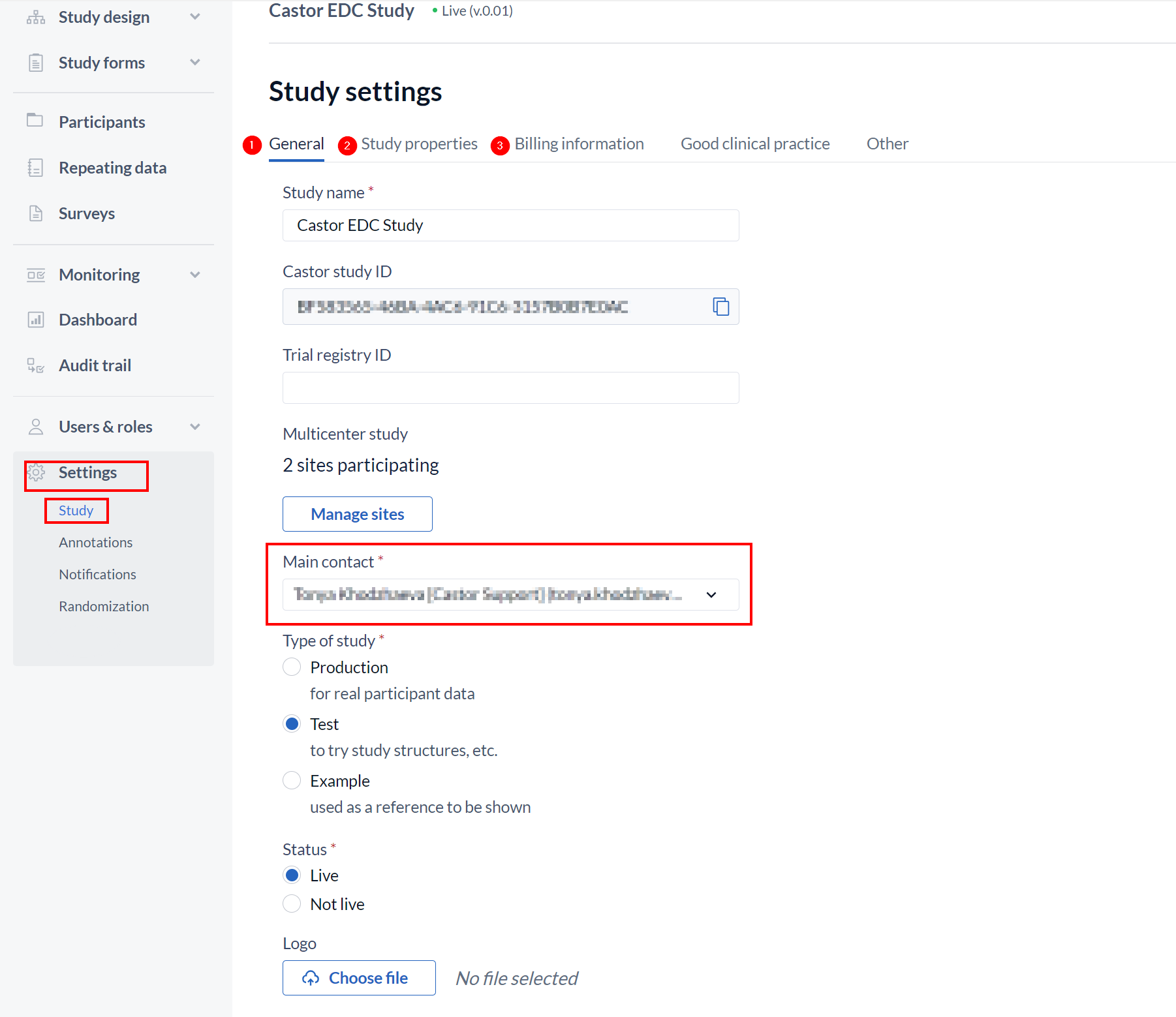
Once the required details are completed, in the section 'Is study live' select 'Live' from the dropdown menu and then click 'Save changes'.
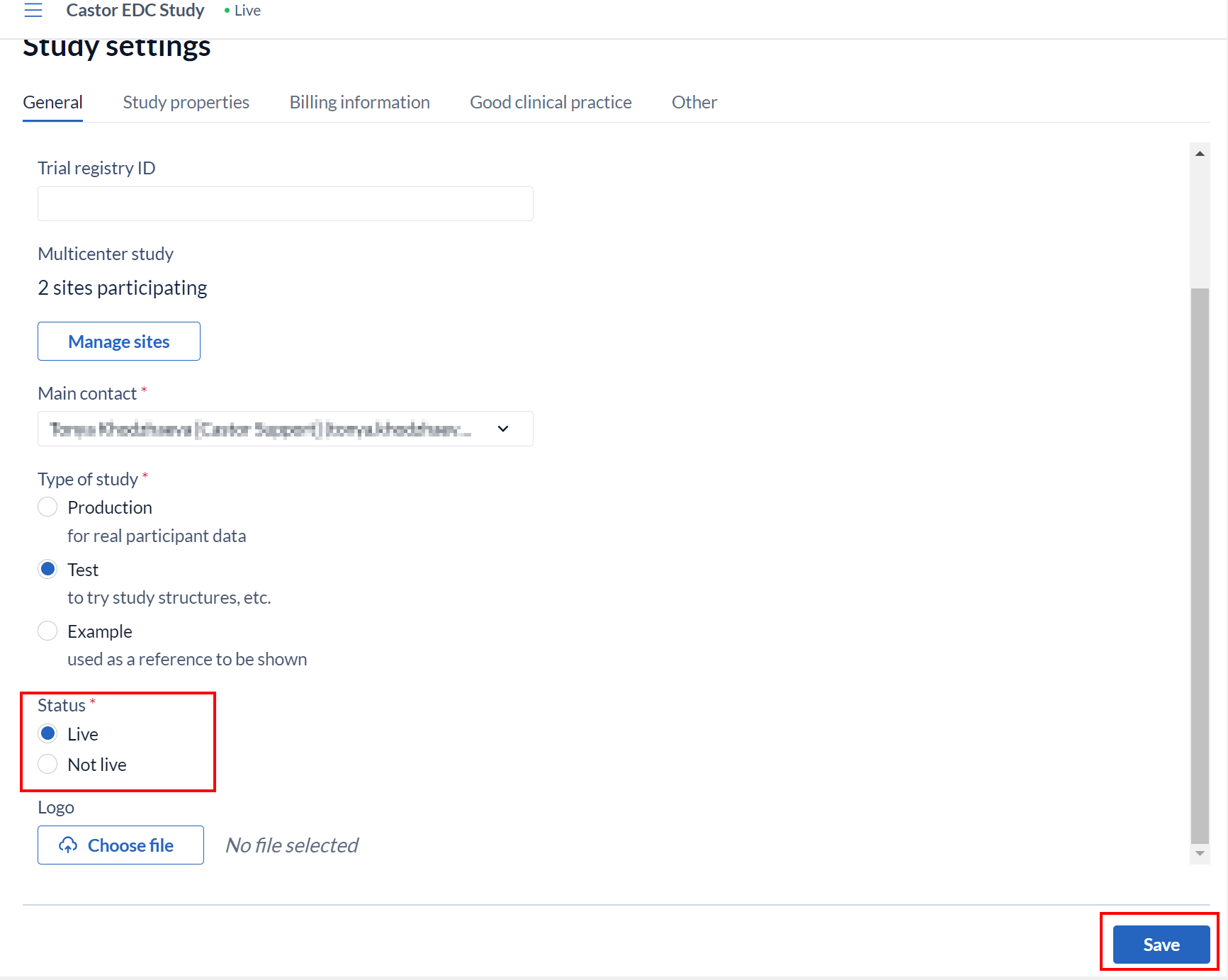
This article describes in detail the consequences of setting your study to live.