Exporteren van studiegegevens
De gegevens in Castor SMS kunnen gemakkelijk naar een Excel bestand geëxporteerd worden.
Ga via het algemene Studies menu naar de 'Export' knop en kies de gewenste export functie.
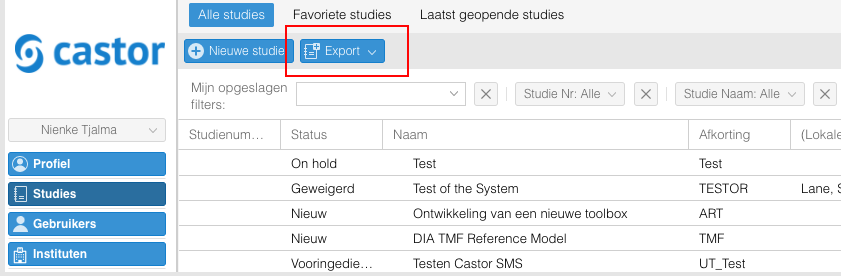
- Een van de voorgedefinieerde export rapporten
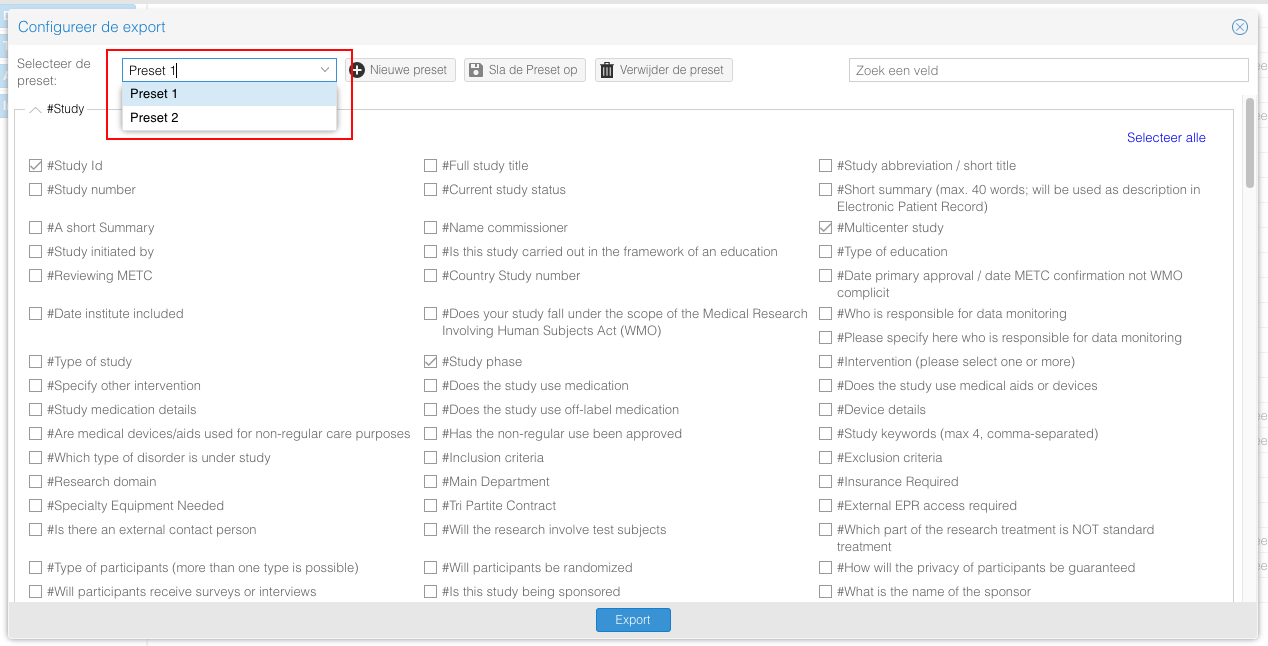
- Een zelf te maken export rapport middels "Uitgebreide export"
- Selecteer de te exporteren velden en klik op "Exporteren" om deze velden te exporteren
- Gebruik de "Nieuwe preset" knop om een preset op te slaan
Filteren
Alle studies waar je inzage rechten voor hebt worden meegenomen in de export. In Excel kun je vervolgens op de juiste studies filteren.