Import the form structure in CDMS
Table of Contents
You can use the exported files to import the forms into another study or copy structures. XML, ZIP or CDISC ODM formats can be used for importing forms.
1. To start importing a structure file, click the 'Import' button.
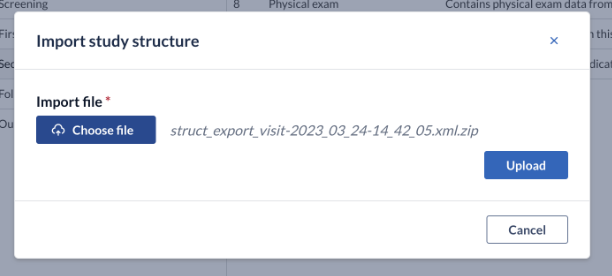
2. Click the 'Choose file' button to select the file (.zip, .xml) containing the structure from your computer's files. Click the 'Upload' button to upload the structure to Castor.
3. If you import an element of which the position matters, dropdown menus will appear where you must select where you want to insert the element. Once a position is selected, click 'Continue import' to continue importing the structure.
If the system detects duplicate variables, the variables will be automatically cleared after pressing the ‘Continue Import’ button. A warning message will appear: We found import elements for which there is a duplicate unique identifier conflict. The system has automatically cleared the IDs for the new imported elements. Please see the the list below before continuing with the import.
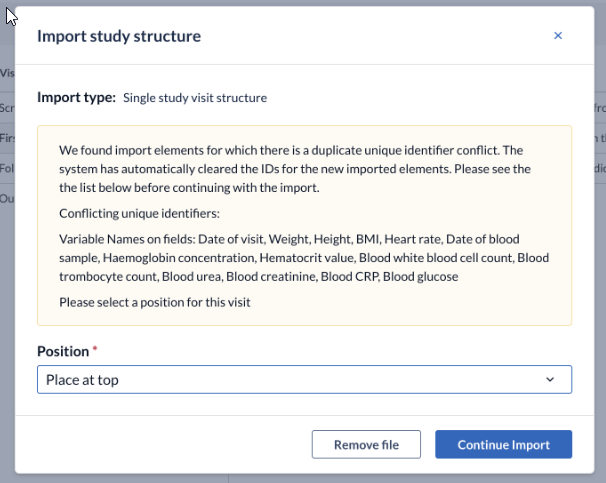
Pressing the ‘Remove file’ button will cancel the import process.
4. When importing a repeating data structure, it is strongly advised to check that all repeating data structures have a unique name, as duplicate names may cause confusion and errors when collecting and exporting the data.
5. When the import from the zip or xml file is successful, you can find your imported elements in the position you specified in the previous form. If the imported items don't appear directly in the interface, re-opening the study should resolve this.
For CDISC ODM format, please consider the limitations which are listed in the article CDISC export and import limitations
Importing the same structure
In some cases it might be necessary to copy the same visit into the same study. In this case all the variable names will cleared since the variable names should be unique across the entire study. This can be solved by copying separate forms into the new visit and selecting the necessary variable prefix, suffix or replacing the variable.
Importing survey packages
If multiple survey packages, that contain the same survey, are imported the result will be duplicates of the same survey form.
To avoid this, you will want to upload the package once. After uploading the package, use the copy option on the cogwheel to copy the package and rename your package and field variable name if needed.