Upcoming Terminology changes in Castor EDC
With the CDMS 2022.3.0 release scheduled for August 2022, we will be introducing terminology changes to better match those more widely used in the industry. By using terms more familiar to our users in Castor EDC, we aim to improve the learning curve and overall experience on our platform.
The API endpoints won’t be modified yet, we’ll announce any new changes to these to all our customers when that happens.
Important note: The new terms have not been changed in the API endpoints and XML files, nor in the keys used within the XML files. We’ll announce any further modifications to these to all our customers when that happens.
We’ve changed the Audit trail event names for all newly created events. The old ones will remain the same, as the audit trail events are stored statically. But don’t worry, if, for a example, you use a filter for a specific event type, it will retrieve all matching events with both the original and the updated terminology. We have also adjusted the column names in the export files.
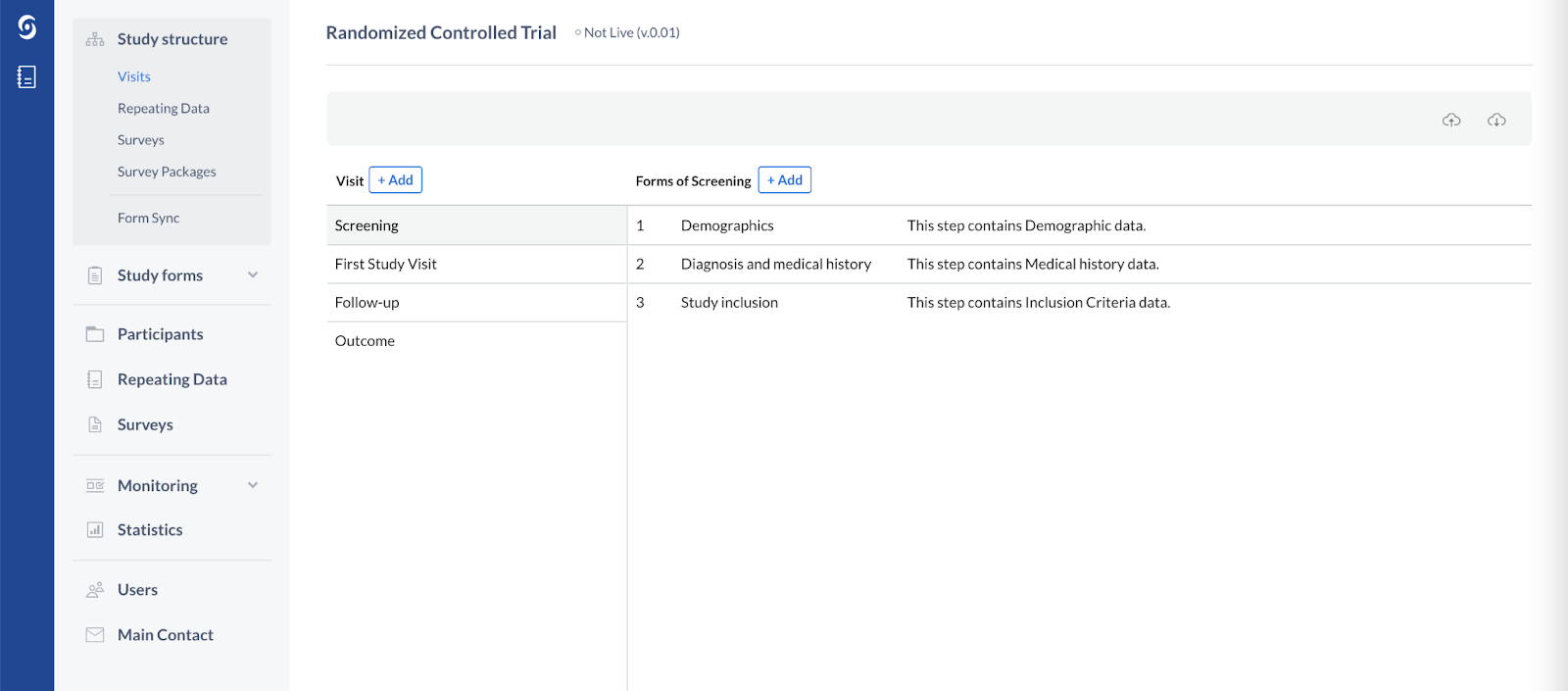
Here’s the summary of the planned changes:
Current term |
New term (as of 2022.3 version) |
Description |
Phase |
Visit |
Phases are periods in a study, e.g. "Baseline", "Visit 1" etc. These can have a specific duration, e.g. 2 months. Only study forms can consist of phases, not reports or surveys. The term ‘Phase’ will be renamed to ‘Visit’. |
Step |
Form |
Steps are sections inside a Study Phase. A Study Phase can have as many steps as you like. Steps break up big forms, and make the CRF user friendly and less bulky. Steps are also present in the reports or surveys. The ‘Step’ will be renamed to ‘Form’ |
Institute |
Site |
Study admins can set up a multicenter study by adding new institutes via the 'Settings' tab in the study. In the 2022.3 version, the term ‘Institute’ will be replaced with the term ‘Site’. |
Record |
Participant |
A ‘record’ in Castor EDC represents all information related to a subject. The term ‘Record’ will be referred to as ‘Participant’ starting from the 2022.3 version. |
Report |
Repeating Data |
Reports are repeating forms and can be used for repeating data such as ‘Adverse Event’ Reports, medication, and measurements such as blood pressure etcetera. They have a “one-to-many” (1:N) relation with the record. In other words, when it is not known in advance how many times a specific measurement will be performed or how often an event (e.g. adverse event) will occur, use reports. In the 2022.3 version, the ‘Report’ will be substituted with the term ‘Repeating Data’. |