Manage study documents (TMF) in SMS
Add documents to a study
- Go to the 'Studies' tab.
- Open the study.
- Go to the 'TMF' tab.
- Click on '+ Documents'.
- Select one or multiple documents that you want to add to the study. Please note that only documents in the following formats can be uploaded: png, jpg, jpeg, gif, bmp, txt, ppt, pptx, xps, eml, msg, xls, xlsx, csv, pdf, doc, docx, docm, odt, rtf, mp4, sps, dat, sav, sql, asp, sas, sas7bdat, xml, xlsb, xlsm, zip.
.png)
- Please be aware that you are not allowed to upload documents which contains sensitive personal data and/or citizen service number. You are also not allowed to upload documents which contains any personal data that can be traced directly or indirectly back to a study participant, such as but not limited to name and hospital information system number.
- Fill in the required fields per study document. Use the 'Next' and 'Previous' buttons to navigate between the document.
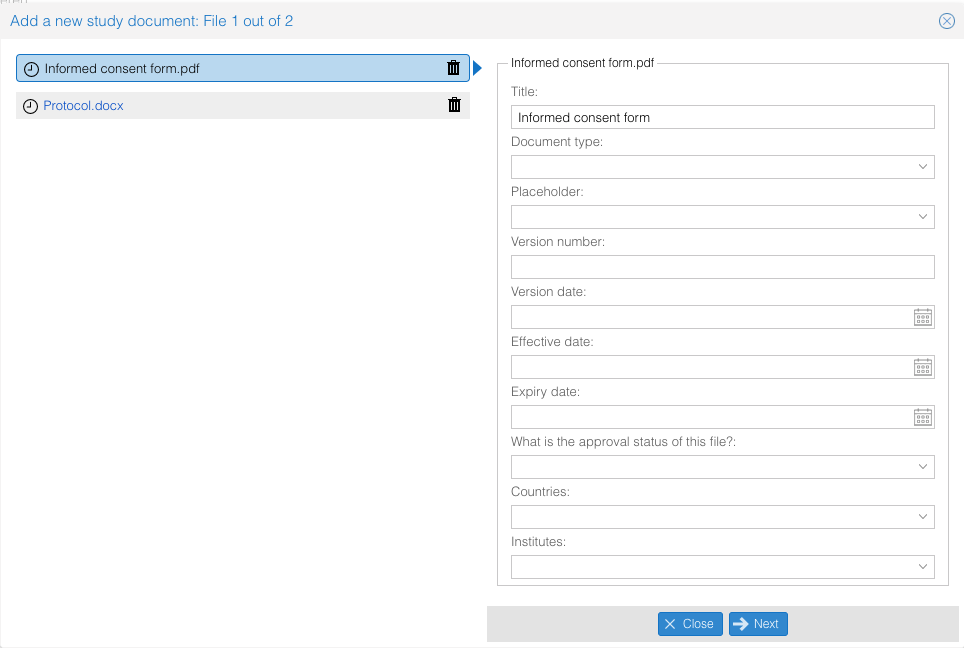 Not all fields have to be filled in. For example: In case you don't want to link a document to a specific country or institute (site), you can leave these fields empty.
Not all fields have to be filled in. For example: In case you don't want to link a document to a specific country or institute (site), you can leave these fields empty.
- Select one or multiple documents that you want to add to the study. Please note that only documents in the following formats can be uploaded: png, jpg, jpeg, gif, bmp, txt, ppt, pptx, xps, eml, msg, xls, xlsx, csv, pdf, doc, docx, docm, odt, rtf, mp4, sps, dat, sav, sql, asp, sas, sas7bdat, xml, xlsb, xlsm, zip.
- Click 'Done' when all required details have been added.
The document(s) are now added to the study in the corresponding folders.
Upload one document to a specific placeholder
- Go to the 'Studies' tab.
- Open the study.
- Go to the 'TMF' tab.
- Go to the desired placeholder and click on 'Upload' or use the right mouse click.
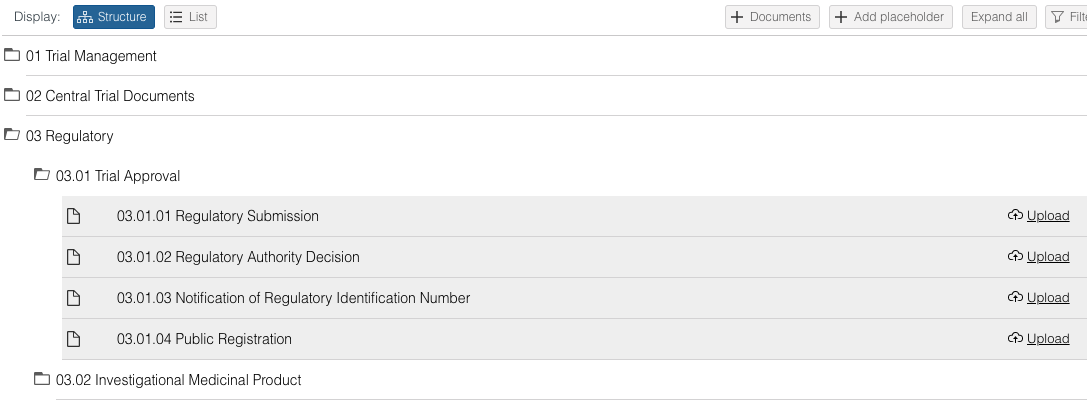
- From here, follow the document upload flow, see above.
The document is now added to the selected placeholder.
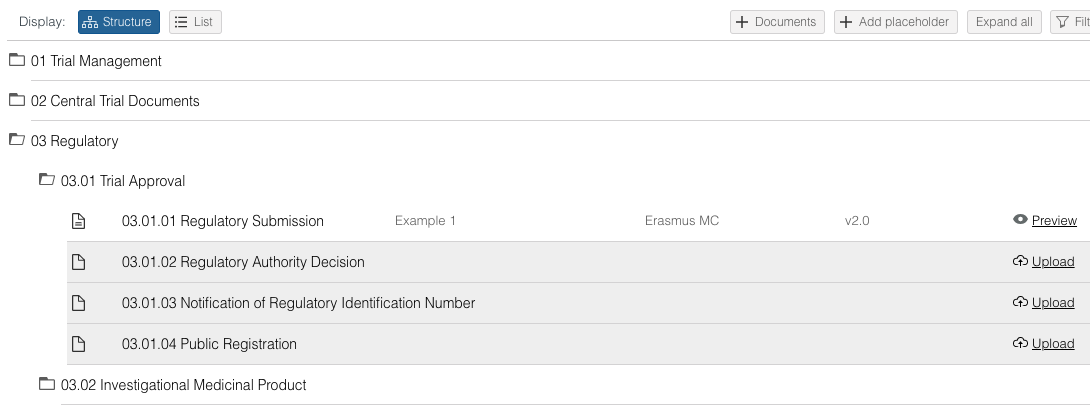
View, download, archive and delete documents via List view
- Go to the 'Studies' tab.
- Open a study.
- Go to the 'TMF' tab.
- Click on 'List' to open the list view
- Use the right mouse click or action icon to select the action.
.jpg)
View, download, archive and delete documents via Structure view
- Go to the 'Studies' tab.
- Open a study.
- Go to the 'TMF' tab.
- Click on 'Structure' to open the Structure (folder) view.
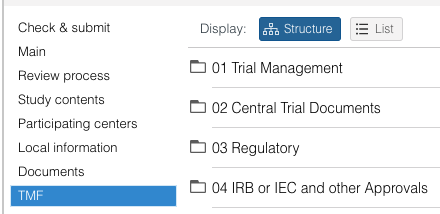
- When clicking once on a document, a drawer on the right side will open that displays the document details. Use the icons at the top of the drawer to download, archive or delete the document.
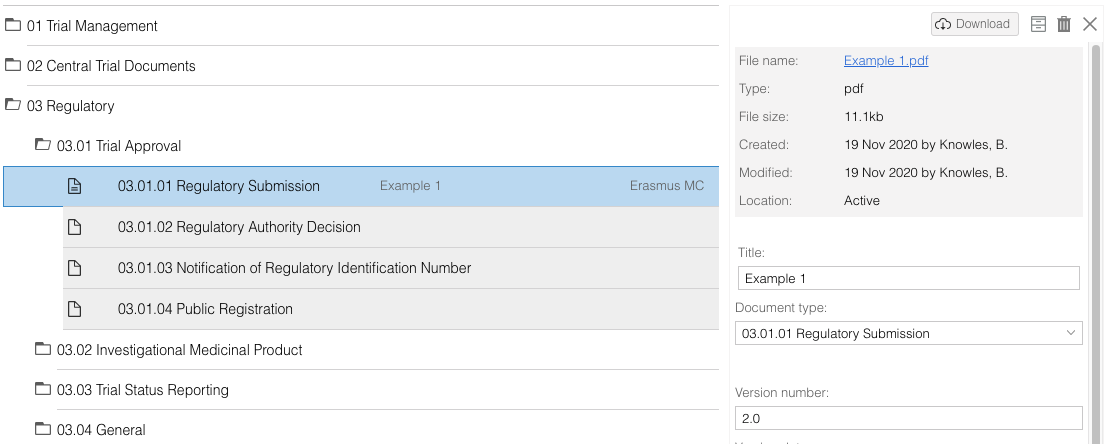
- When clicking twice on a document, a detailed view for that document will open. Use the icons at the right top to download, archive or delete the document. There is a navigation on the left side of the screen to go to the document preview of document specific audit trail.
Find specific study documents
- Go to the 'Studies' tab.
- Open a study.
- Go to the 'TMF' tab.
- Click on 'Filter' to open the filter menu.
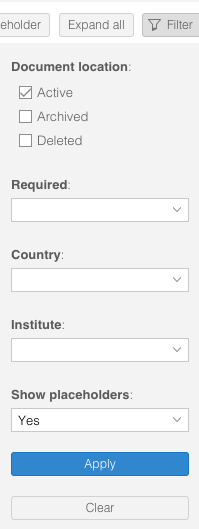
- Select the desired filters and click 'Apply' to activate the filters.
- Document location: Show the active, archived and/or deleted documents
- Required: Only show the document types that are required in this study status
- Country: Only show the documents that are linked to specific countries
- Institute: Only show the documents that are linked to specific institutes (sites)
- Show placeholders: Show placeholders or only the documents