Request a quote from a participating/supporting department in SMS
When requesting a quote from a participating/supporting department, it is important to add the number of requested operations/procedures first.
Adding a participating department
- Go to the 'Studies' tab.
- Open the study.
- Go to the 'Participating departments' tab.
- Click on 'Add participating department'.
- Complete the form.
- Details: Fill in the general information.
- Operations: Add the number of operations/procedures that are needed from the department. State how many are part of regular care, and how any are part of the study.
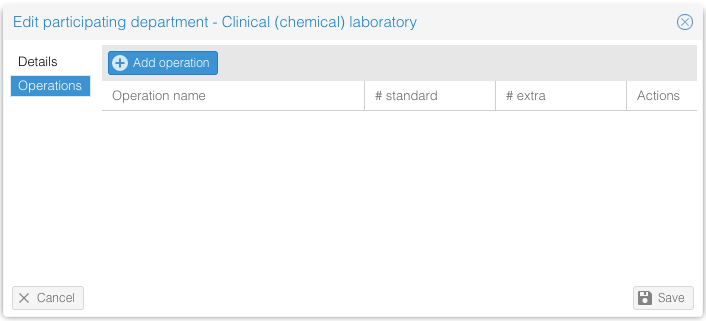
- Click on 'Save' to add the participating department.
The participating department is now added to the study and users with the user role of this department can view this study.
Request a quote
- Use the right mouse click to select 'Request offer', or click on the envelope icon in the row.
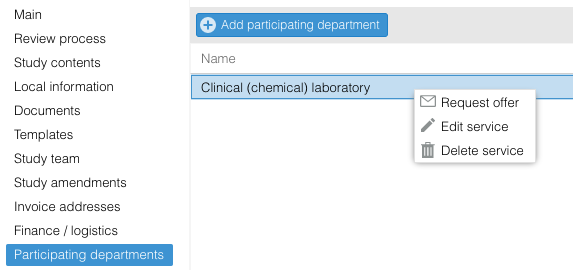
An email will be sent to all users with a role in this department.