Repeating Data overview in CDMS
In the 'Repeating Data' tab, an overview of all the Repeating data instances within the study is shown. Please note that;
- Users can only view or print Repeating data from participants that belong to a site for which they have "View" rights.
- Users can only (un)archive repeating data Repeating data from participants that belong to an site for which they have "Archive" rights.
Here is an illustration of the Repeated data overview:
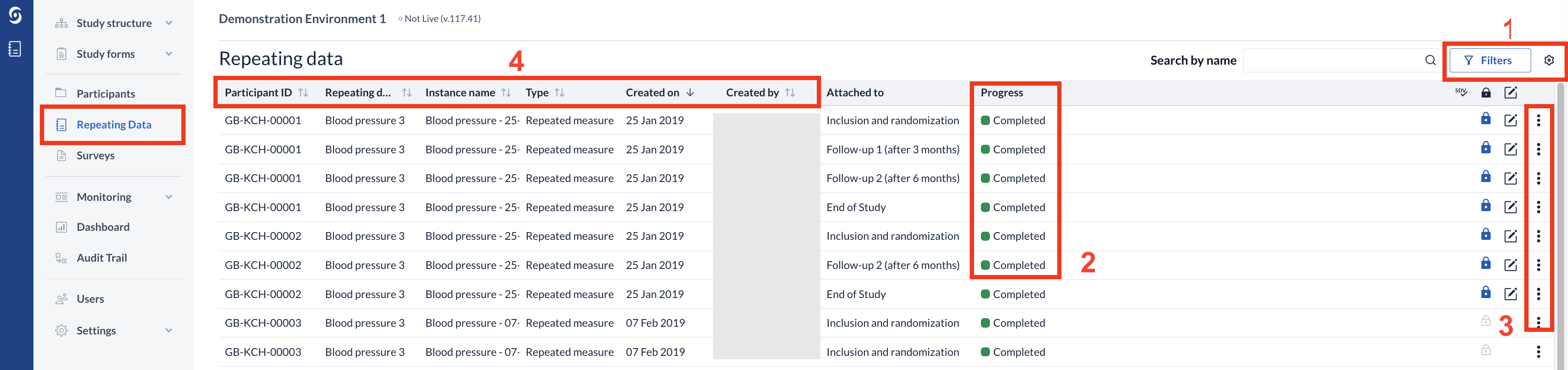
- The 'Filters' button allows users to further refine their search by selecting options such as site, repeating data, visit, status, and completion level. These filters can be used in combination to narrow down the results and provide more specific data. Study admins can configure up to 5 custom columns for the global repeating data overview. These custom columns are shown in the global repeating data listings and can be selected or unselected from the cogwheel menu. If a selected field does not apply or is empty, it will be left empty. If data is blinded per user role, the values will be hidden accordingly.
- An overview of all Repeating data forms is shown, with the completion levels depicted by colour codes and wording. Other details such as name, date and creator are also included. You can double click on a Repeating data instance in the list to be redirected to that instance's data entry view.
- You can sign, archive, unarchive, delete (for non-live studies) or print a Repeating data form using the three dots menu (depending on the user rights). Non-eligible forms (i.e., with pending queries or hidden for the user's role) are skipped when performing the action from the global Repeating data overview.
- By clicking the arrows next to the column headers, you can select by which column to sort the forms in ascending or descending order. Only one column at a time can be used as sorting criteria.
- The overview also displays the Lock, SDV, and Signature status of the repeating data forms. Repeating data instances that are SDV’ed will display the SDV icon, while locked instances display the lock icon. Forms that have been signed will display a signed icon. Repeating data instances that are neither SDV’ed, locked, nor signed will show no icons as illustrated below:
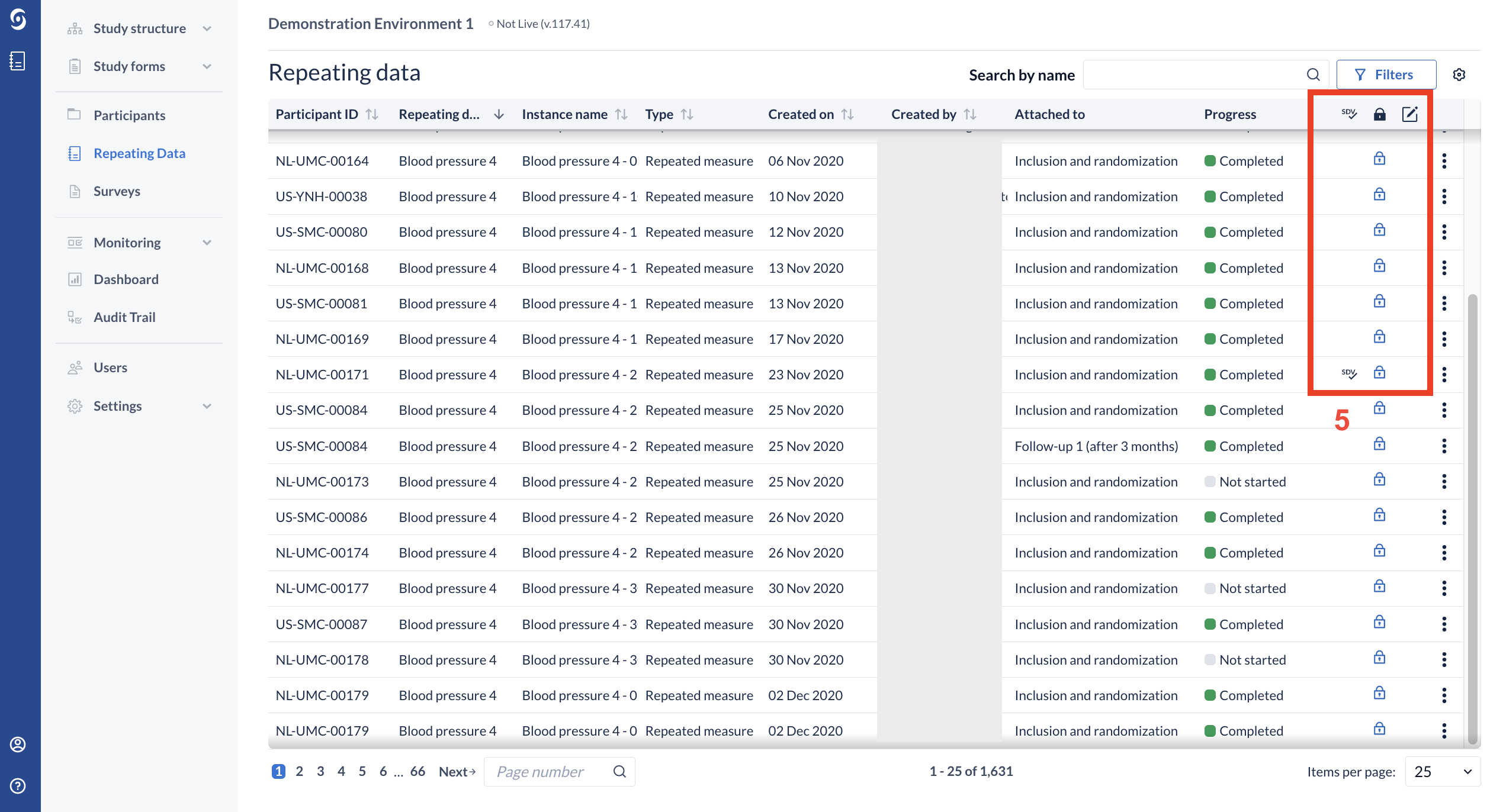
- The column width as well as the column visibility is persistent for the user when navigating away from the page during a continuous session.
- To display archived items directly, use the ‘Filters’ button and select the ‘Archived’ status. The archived repeating data instances have a background color for increased visibility.