Creating and Managing Source Data Verification (SDV) Plan
Table of Contents
Source Data Verification (SDV) is a method used in clinical trials to ensure the data being collected is accurate. It primarily involves a comparison of the source data against its final case report form and aims to identify any errors before study closure.
SDV Plan Capabilities
In Castor CDMS, it is possible for study admins to create SDV Plans and specify fields which require source data verification (SDV).
- Studies can define multiple SDV plans and assign maximum 1 plan per site. Multiple sites can share the same plan, but one site cannot have more than 1 SDV plan.
- An SDV plan will contain the user-defined list of data points (fields) that will be requiring source data verification.
- Some field types are excluded from this view, as those can never be SDV’ed (non data points). These are: Image, Calculation, Add survey, Add Repeating Data, Link, Remark, Summary, QR code, Repeated measurement, Randomization
- Study admins can create and manage multiple SDV plans, but it's important to note that all participants from a site will have the same SDV requirements. This ensures a consistent distribution of SDV across the site's participants.
- Having a plan without any data points assigned to sites or a site without any SDV plan will be considered as without any needs for source data verification. Consider publishing an empty SDV plan (without any fields selected) if you want to be explicit about the lack of SDV requirements for specific site(s).
- In this case, a form, visit or repeating data instance will be marked as verified only when all of its fields have been verified individually
An SDV plan will be applied to each participant's CRF, accounting for availability and visibility rules as configured in the study's settings, structure and/or automation engine (role blinding, dependencies. In this case, a form, visit or repeating data instance will be marked as verified only when all fields requiring SDV have been verified individually.
Users will still be allowed to SDV fields outside and beyond the defined SDV plans.
Creating SDV Plan
To create an SDV plan:
1. Navigate to the Monitoring tab and select the SDV Plan sub tab:
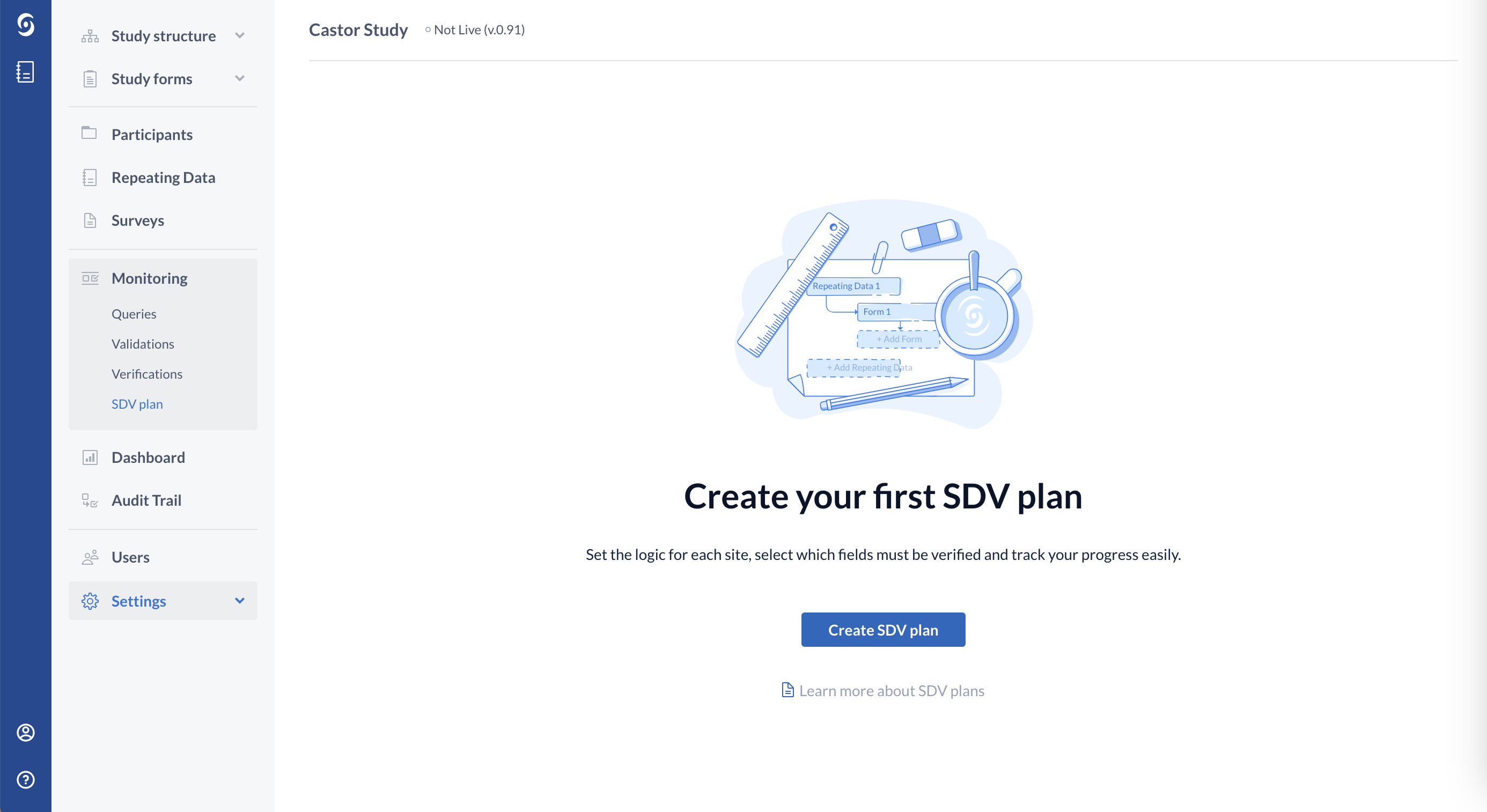
2. Click on the "Create SDV plan" button
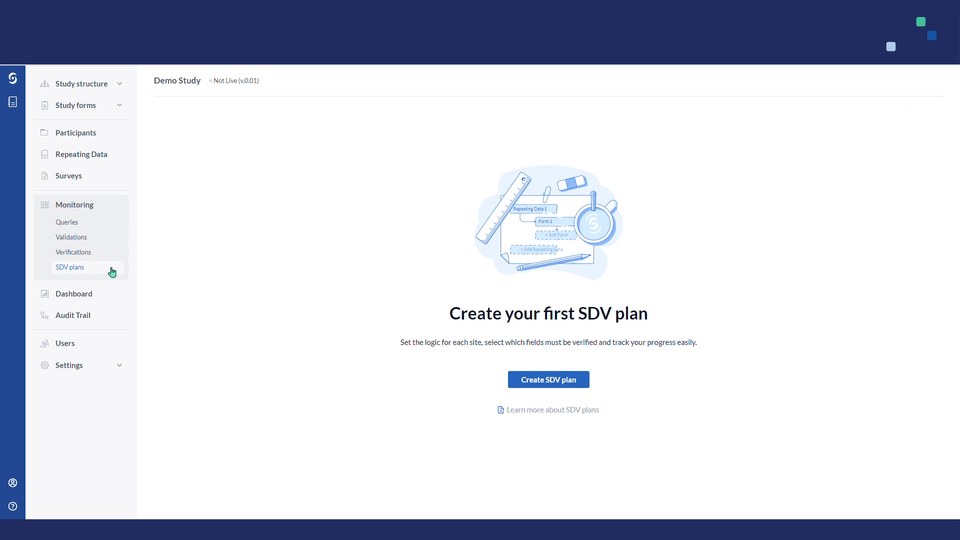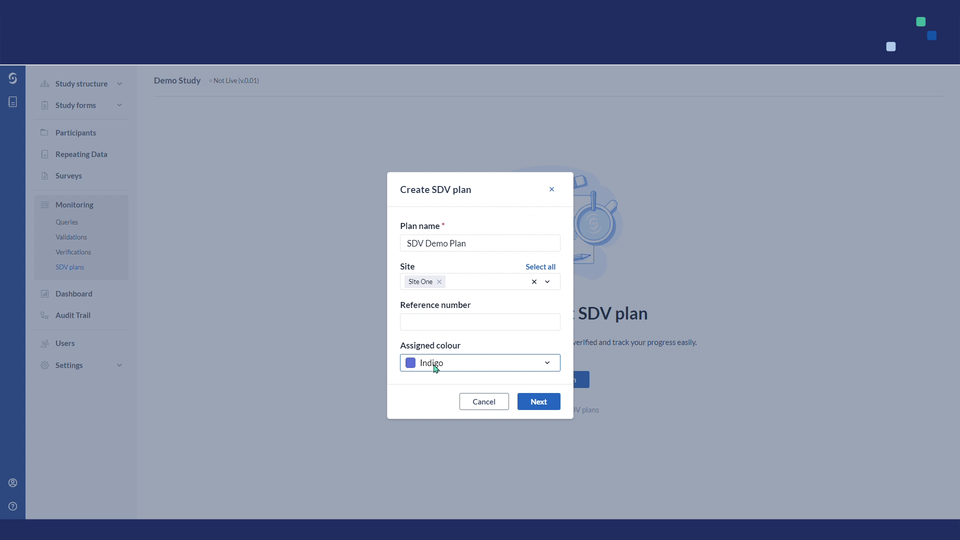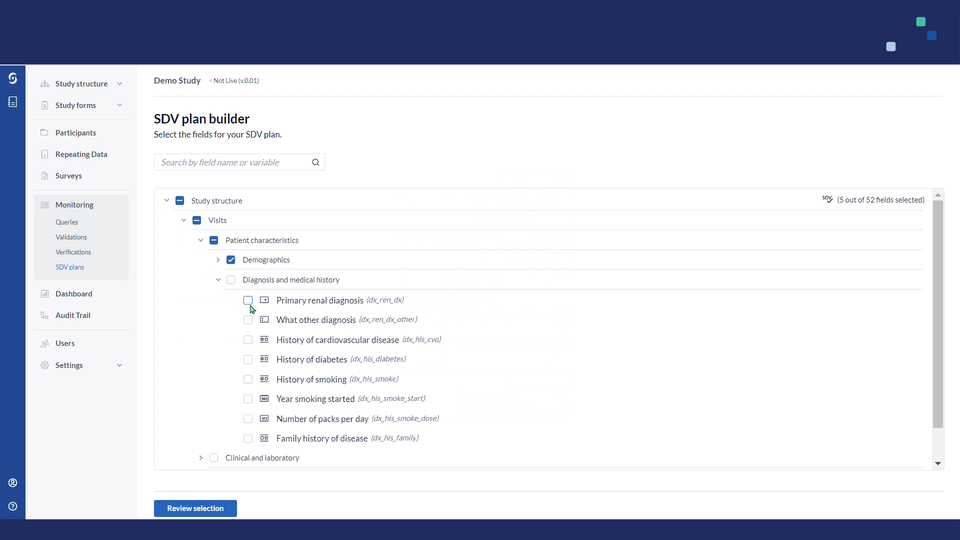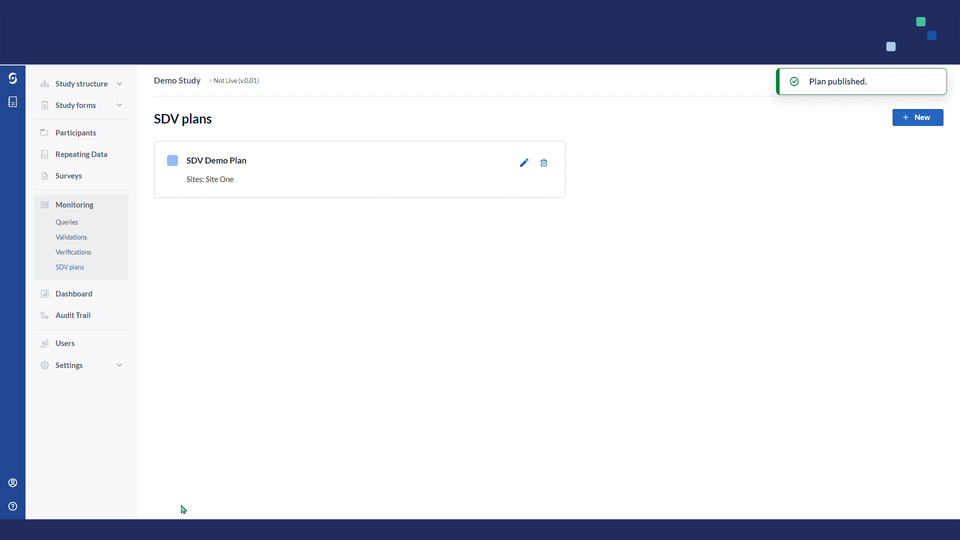
3. Fill out the following details:
- Plan name - title for your SDV plan
- Site - choose the list of sites to which the SDV plan will be linked
- Reference number - add reference number
- Assigned Colour - select colour
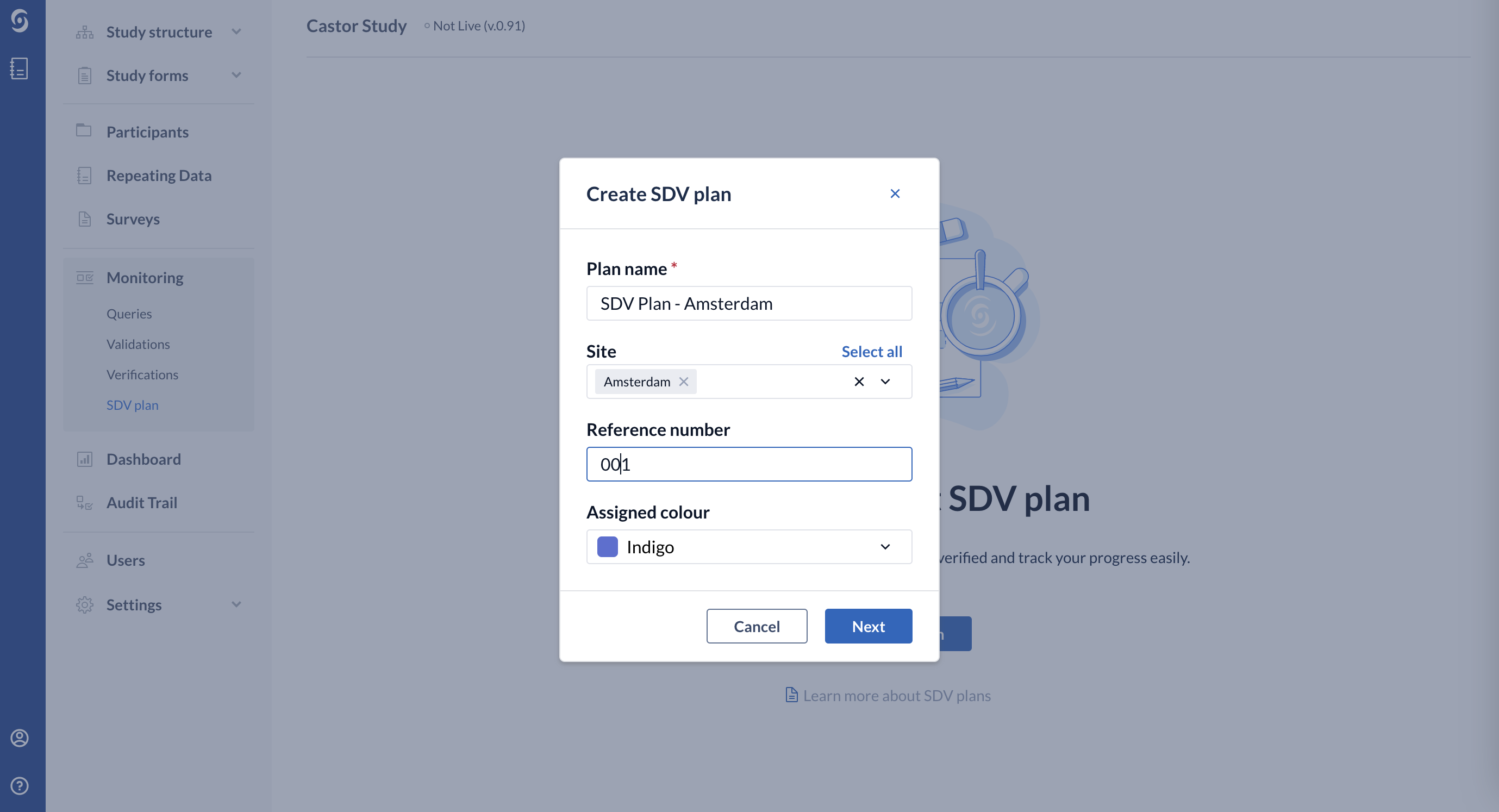
4. Click "Next" to proceed. Afterwards, you will be redirected to the SDV Plan Builder page:
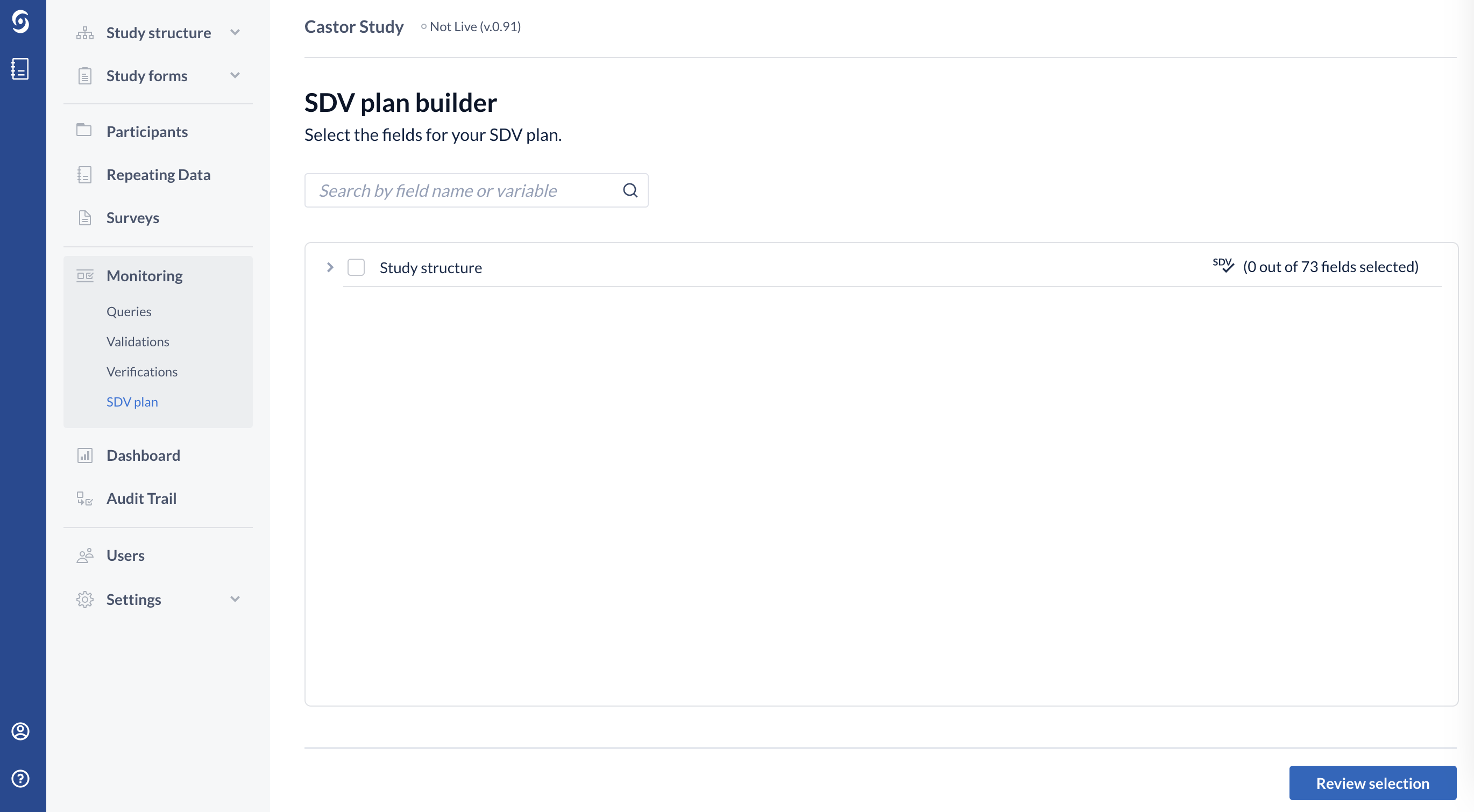
5. Select the fields for your SDV plan by using a search bar or by clicking on the study structure and manually selecting the fields for SDV:
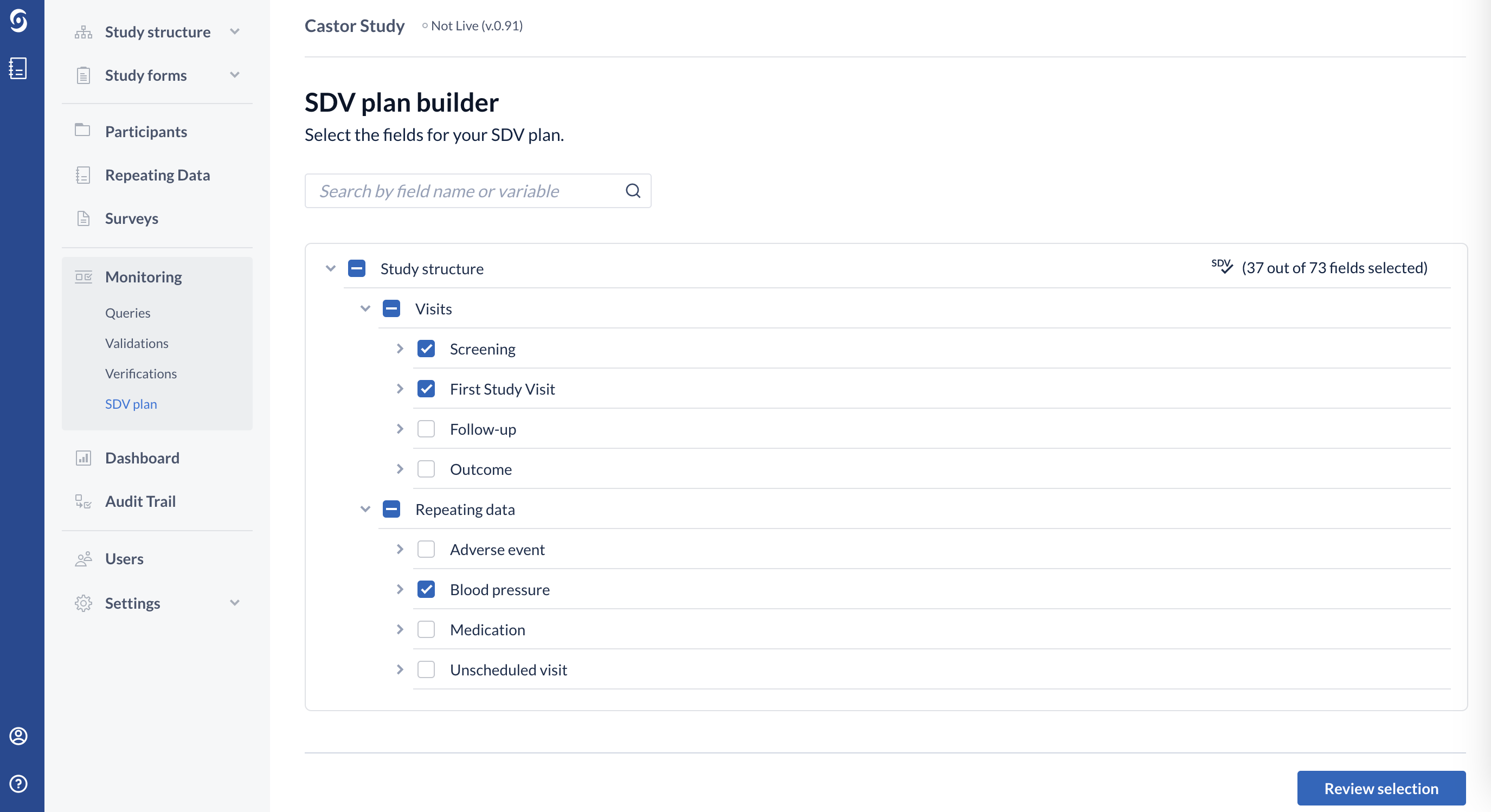
6. Once the fields are selected, click on the "Review selection" button to proceed.
7. In the Review selection page, you will be able to review your SDV information and fields before publishing the SDV plan:
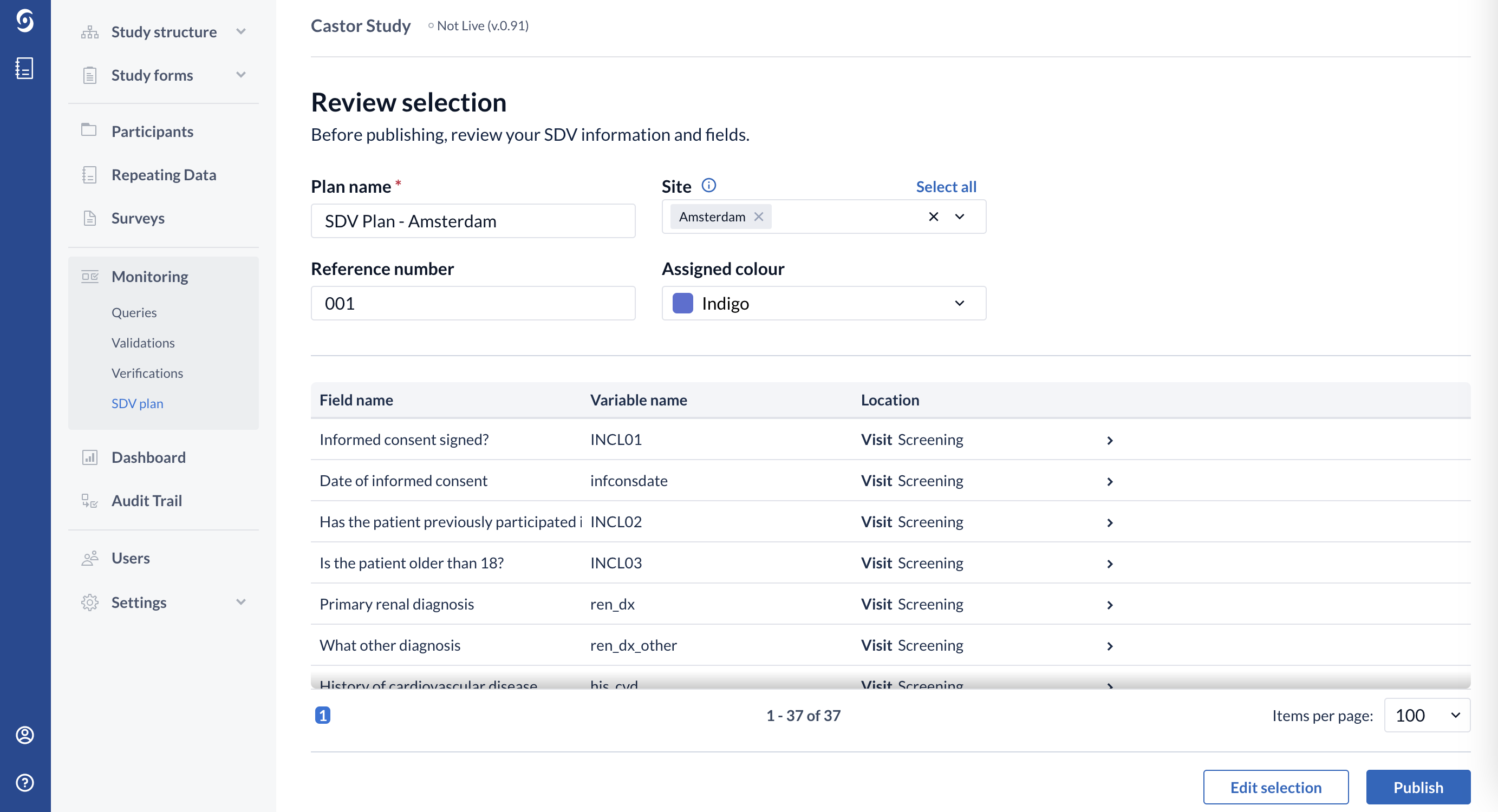
8. Click on "Edit selection" to return to the previous page and adjust the fields for SDV.
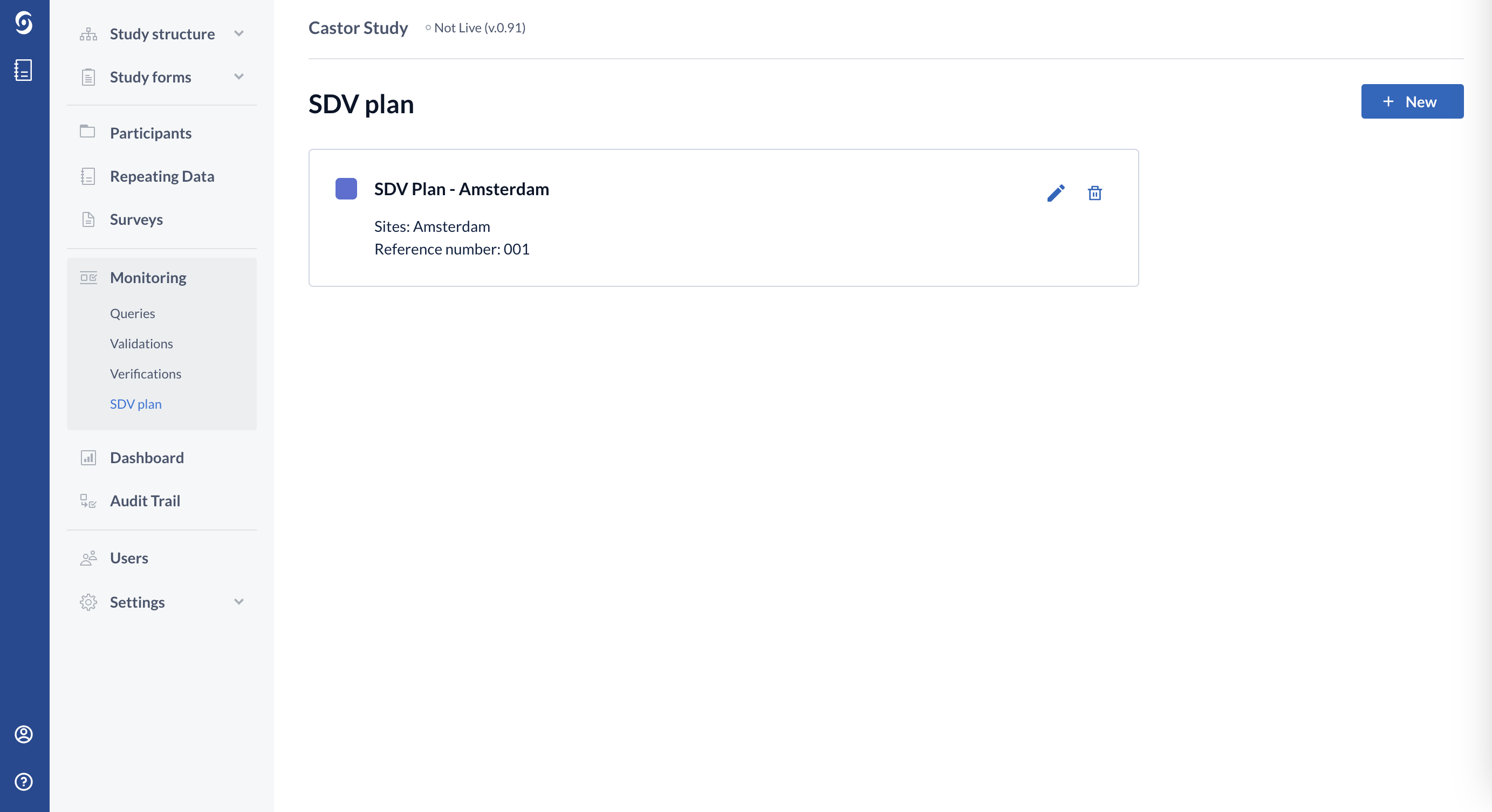
9. When ready, click "Publish" button to publish the SDV plan. Once published, it will be shown in the "SDV plan" page:
Applying SDV in a participant's CRF
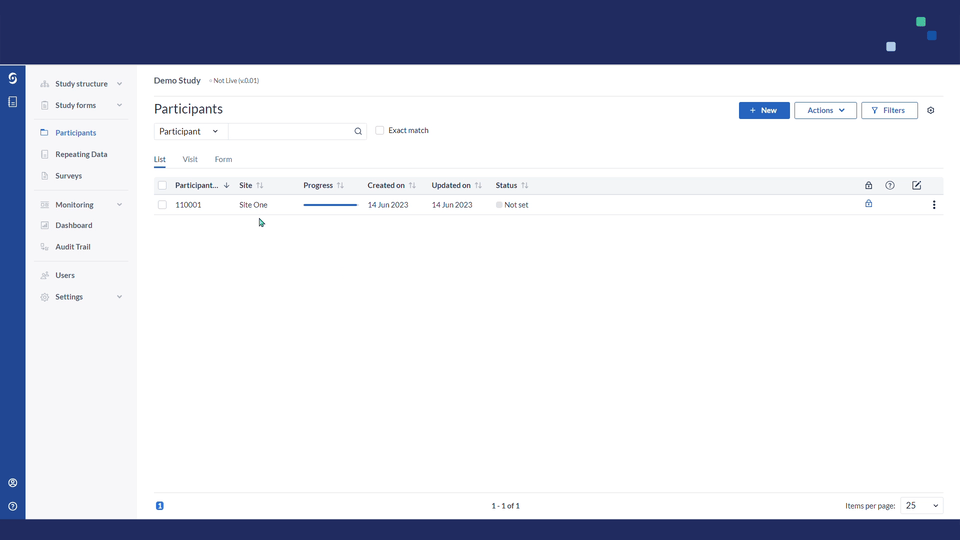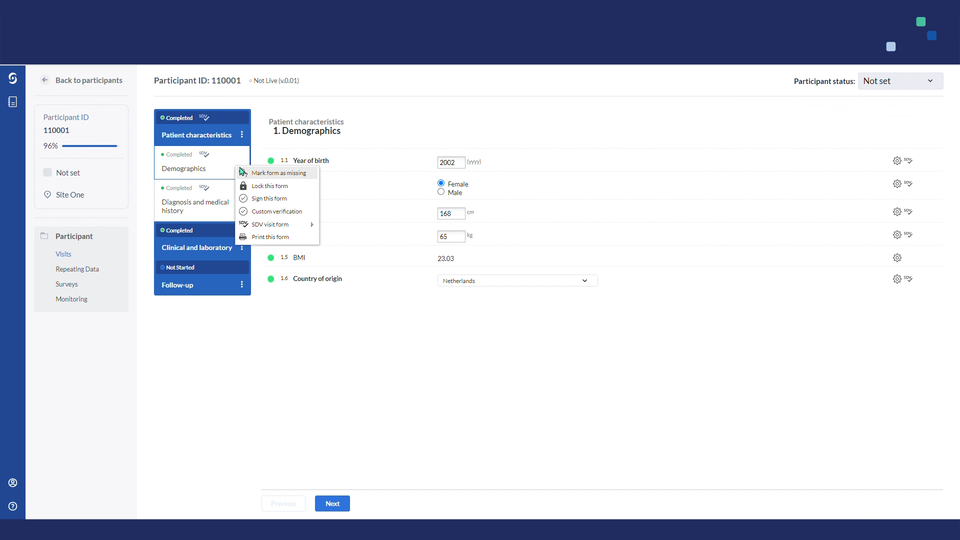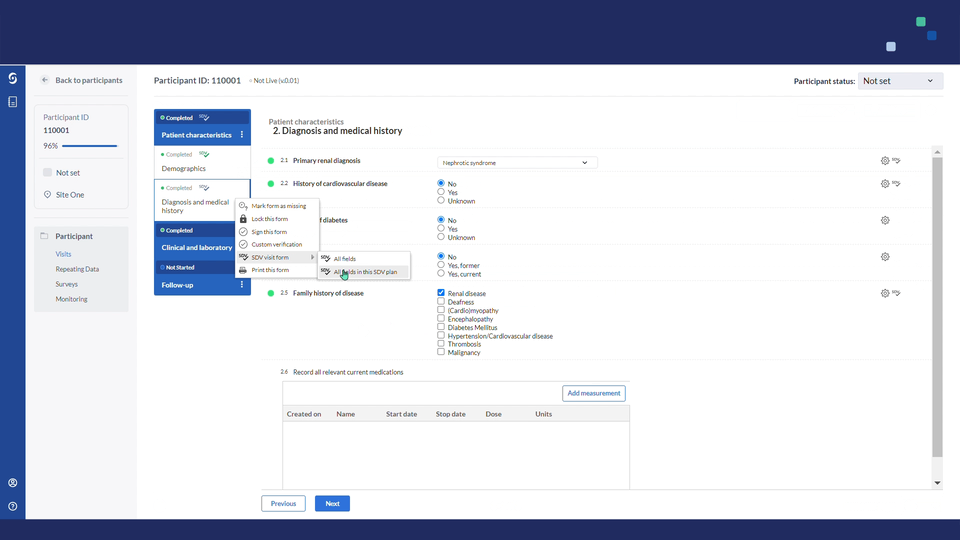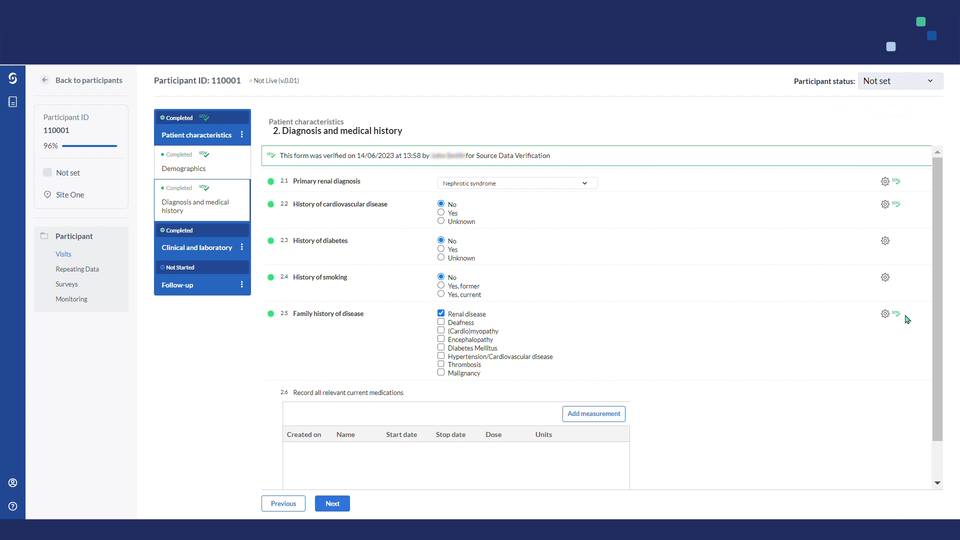
In data entry views, all visible & available fields that are part of that site's SDV plan will display a gray SDV icon.

Once verified, the icon will turn green.
-
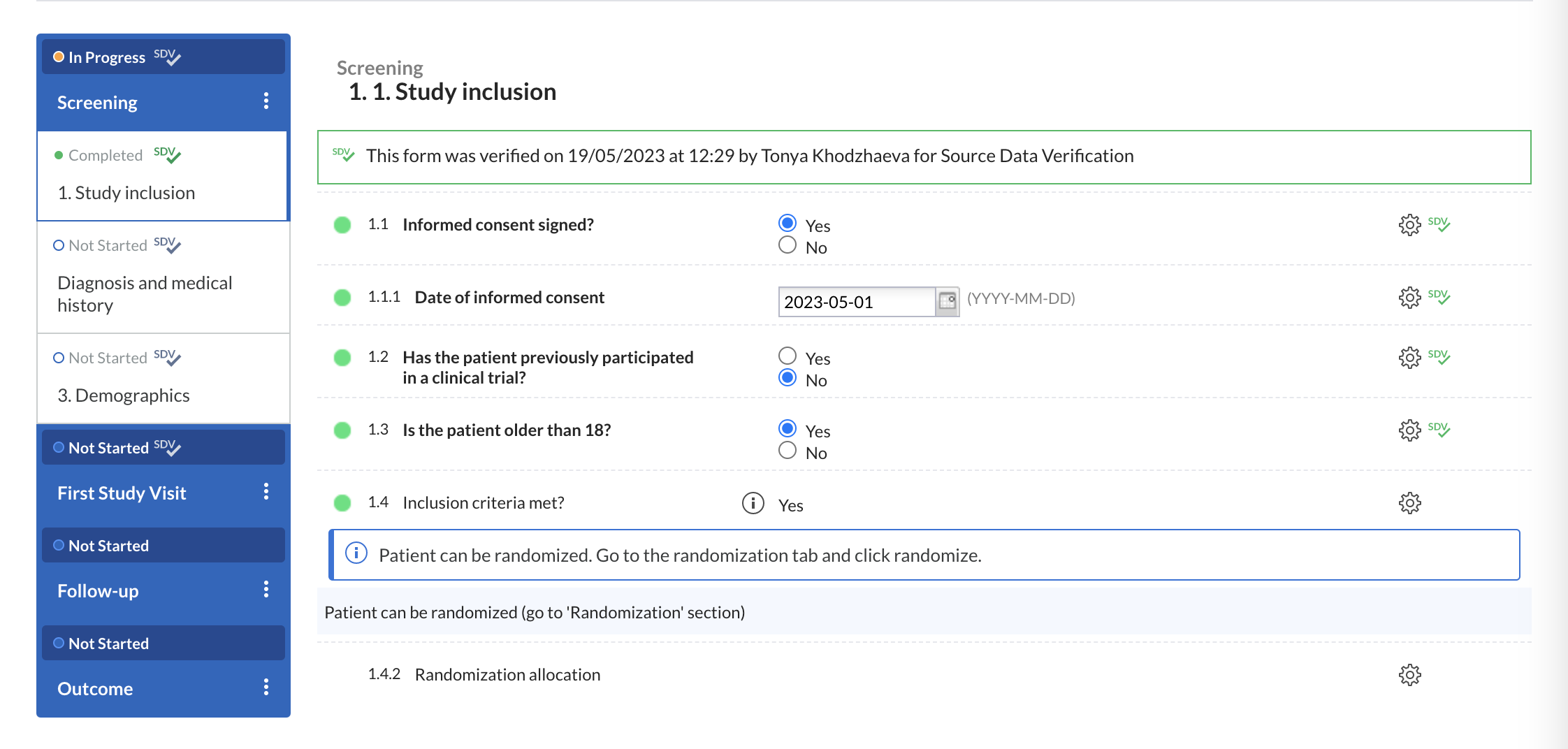
- The same logic has been extended at form, visit and repeating data levels.
- If a form, visit or repeating data instance has no fields requiring SDV, the green SDV icon will be added only after all its fields that can be verified have been verified.
- This optional and additional verification will not influence the SDV progress of your study, as this will only account for the defined SDV plans.
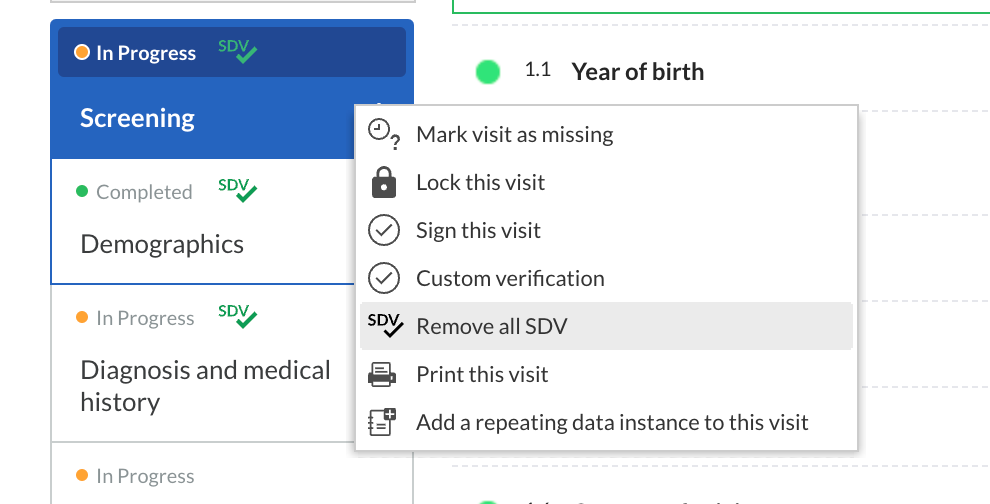
- Users will be able to verify each field individually or multiple fields in bulk.
- Using the cogwheel menu on form, visit or repeating data instance levels, you can choose to verify in batch all fields or all fields in the assigned SDV plan. Once a form, visit or repeating data instance has been marked as verified, you can easily remove all source data verifications in bulk: