Randomize a participant in CDMS
Table of Contents
To be able to randomize participants, randomization must have been first enabled and set up. See Randomization settings for further information on how to set up the randomization for your study. We also have a workshop video on how to randomize a participant.
Open a participant (that needs to be randomized)
Once you have set up your randomization, you can start randomizing participants. In the participants tab, click on the participant you want to randomize.
You can also double-click the participant to enter it. If you have not yet created a participant, learn how to do that here.
Randomize the participant
1. Once in the participant view, click on the 'Not randomized' tab from the left menu:
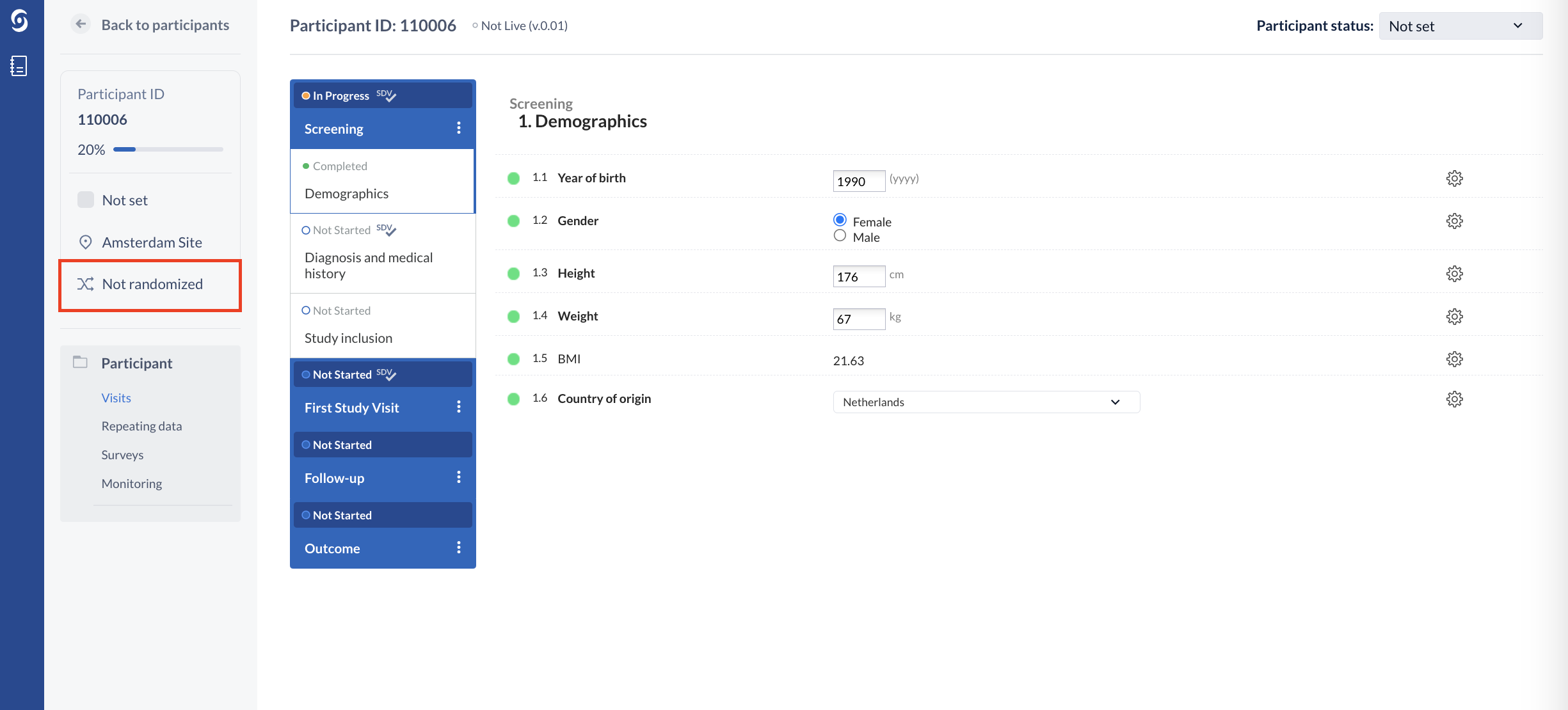
If the participant is excluded, the Randomize button is not available to the user and an informative text is being presented regarding this. For the users without randomization rights, the button will appear as ‘Hidden’.
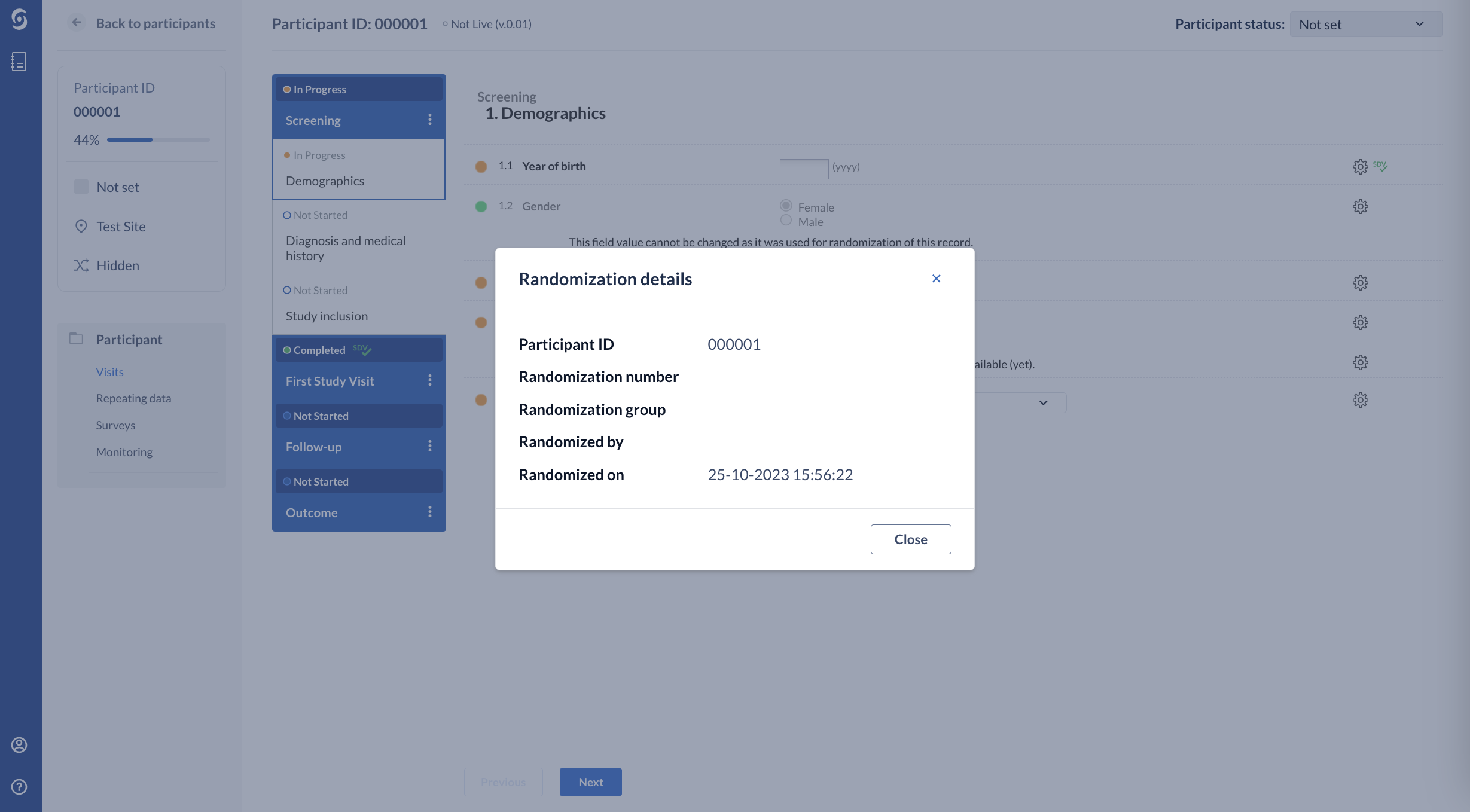
2. A pop-up window ‘Randomization details’ will appear:
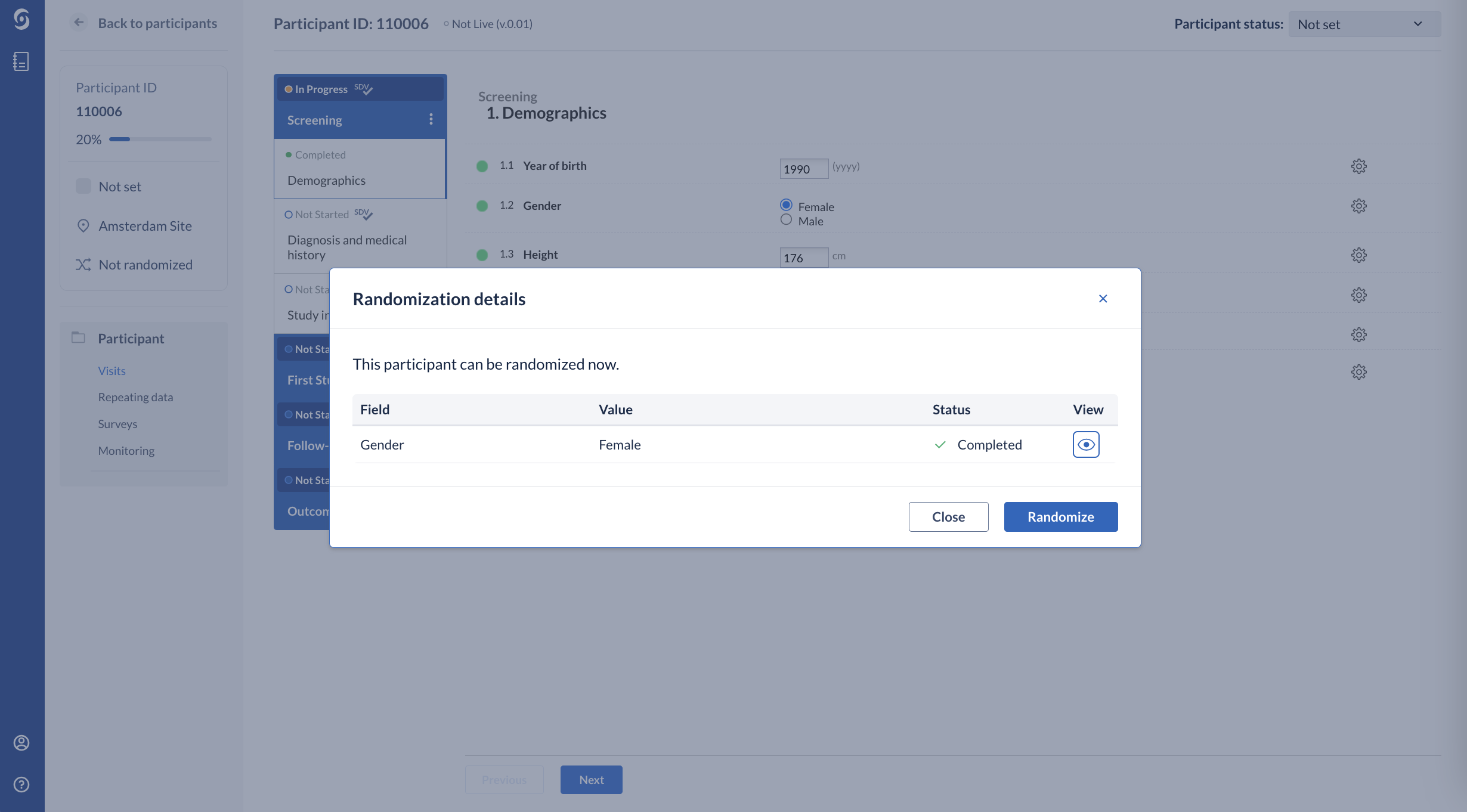
When you have configured randomization that is stratified on one of the data points (fields) in your study, you will see those fields listed in the ‘Randomization details’ window. You can only randomize your participant after all fields that are used for stratification have been completed. For easy access to the set field you can click the eye next to it.
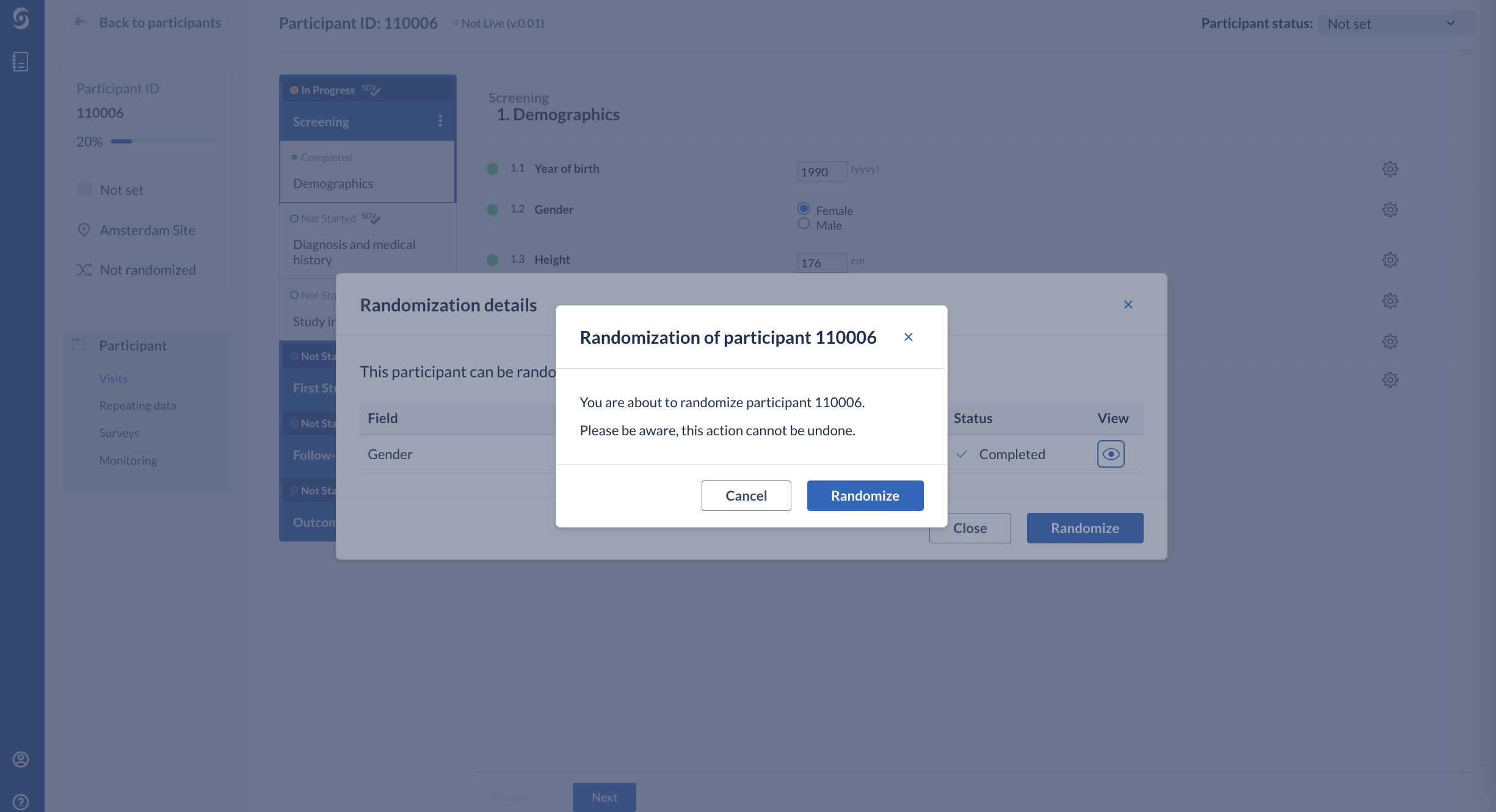
3. Click on ‘Randomize’ button to randomize a participant. After randomizing a pop-up will ask you to confirm the randomization. After randomizing the participant this cannot be undone.
4. The ‘Randomization details’ tab now shows the randomization group and the randomization number and other relevant information:
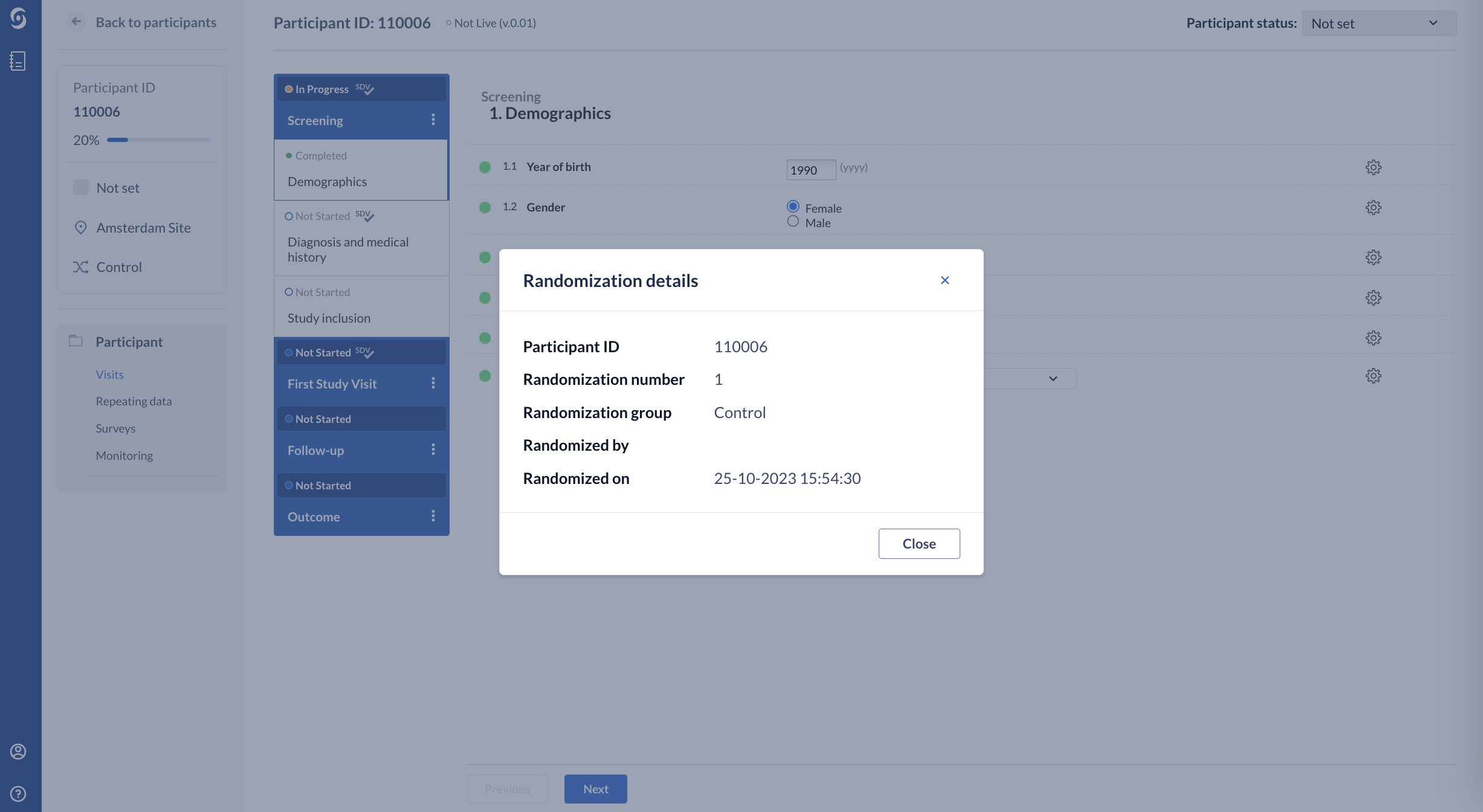
The randomization number is the position of this randomized participant in each randomization group. So the first participant to be randomized in one group will always have randomization number 001, and the second 002 etc.
For users without randomization rights, the randomization number and randomization group fields will not be visible:
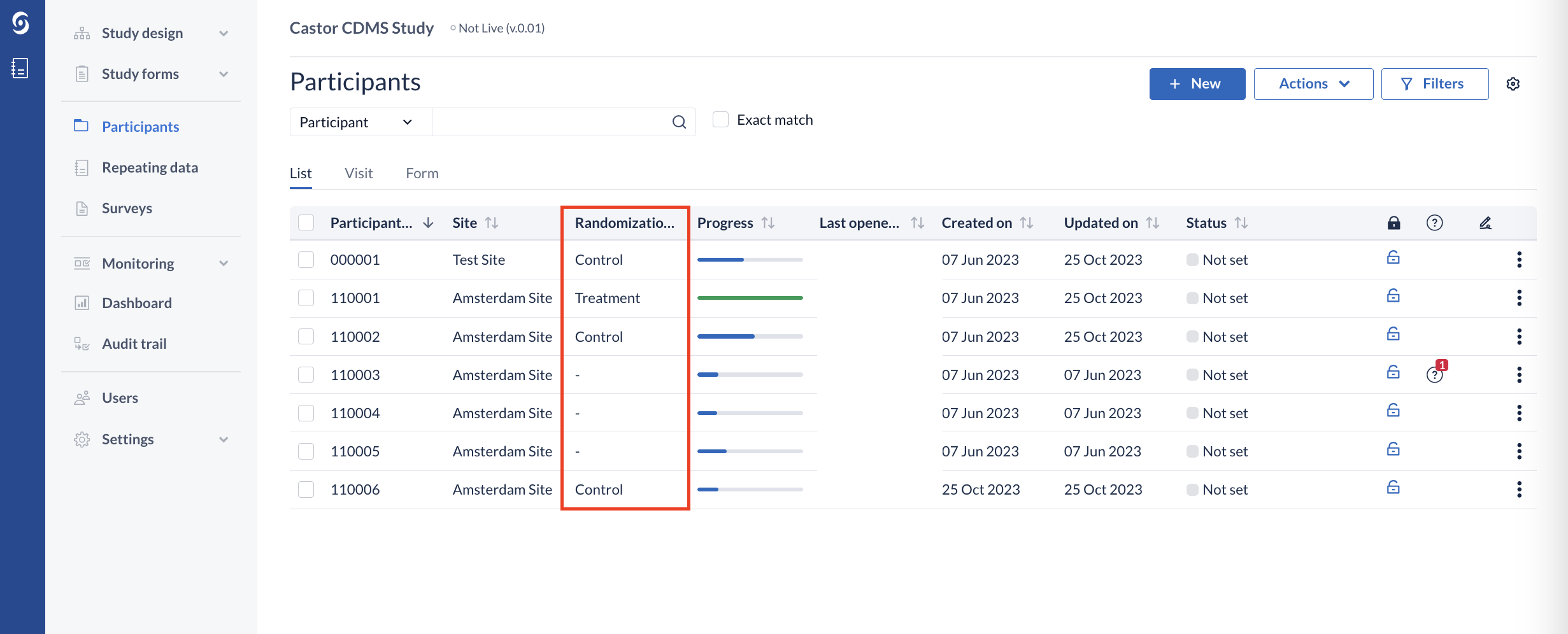
The randomization group is also visible in the participant overview.
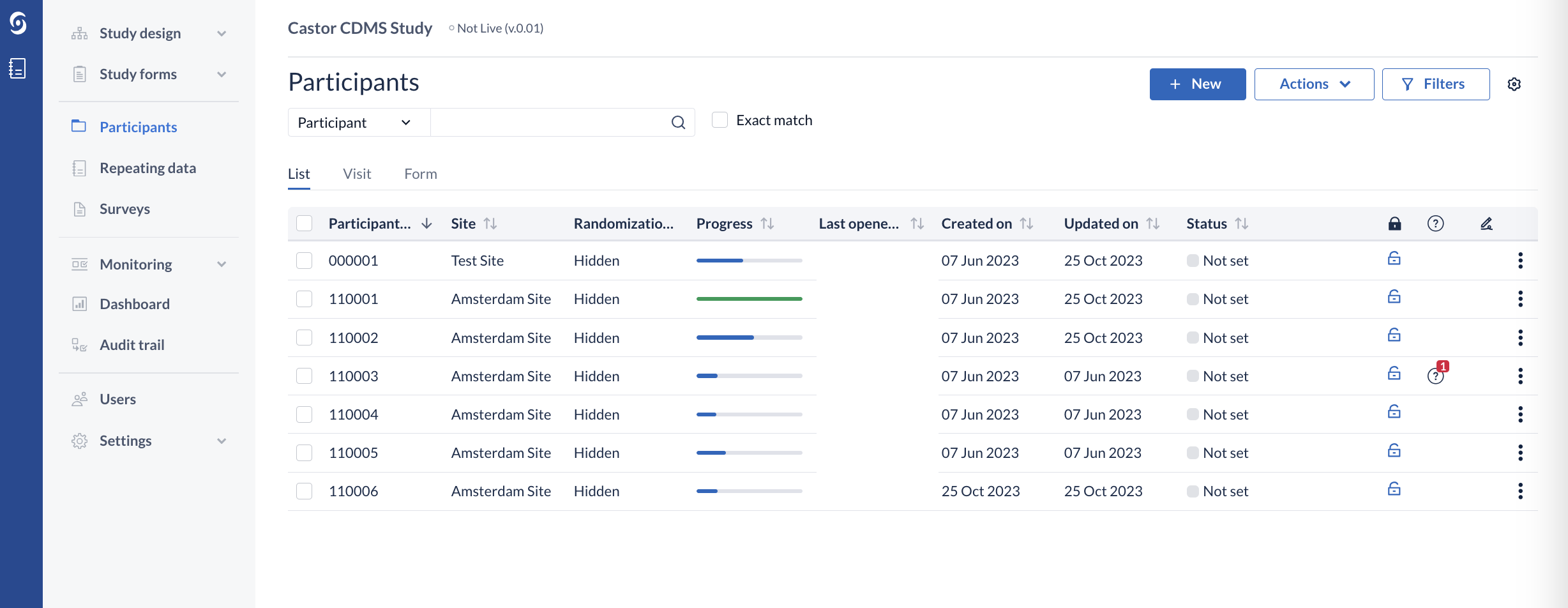
This will not be visible for study users without randomization rights (which allows for blinding) - the group will be shown as 'Hidden'.
When a participant has been randomized, it is no longer possible to change the data for a field or its parent if it has been used for stratification.