Using Repeating Data for Protocol Deviations in EDC/CDMS
Repeating Data structure for Protocol Deviations
Repeating Data structures have a 'one to many' relationship with a participant. Because of this, repeating data structures are useful for collecting information about deviations. It is recommended that the ‘Repeating Data button’ is utilized and dependencies are created where a deviation may occur.
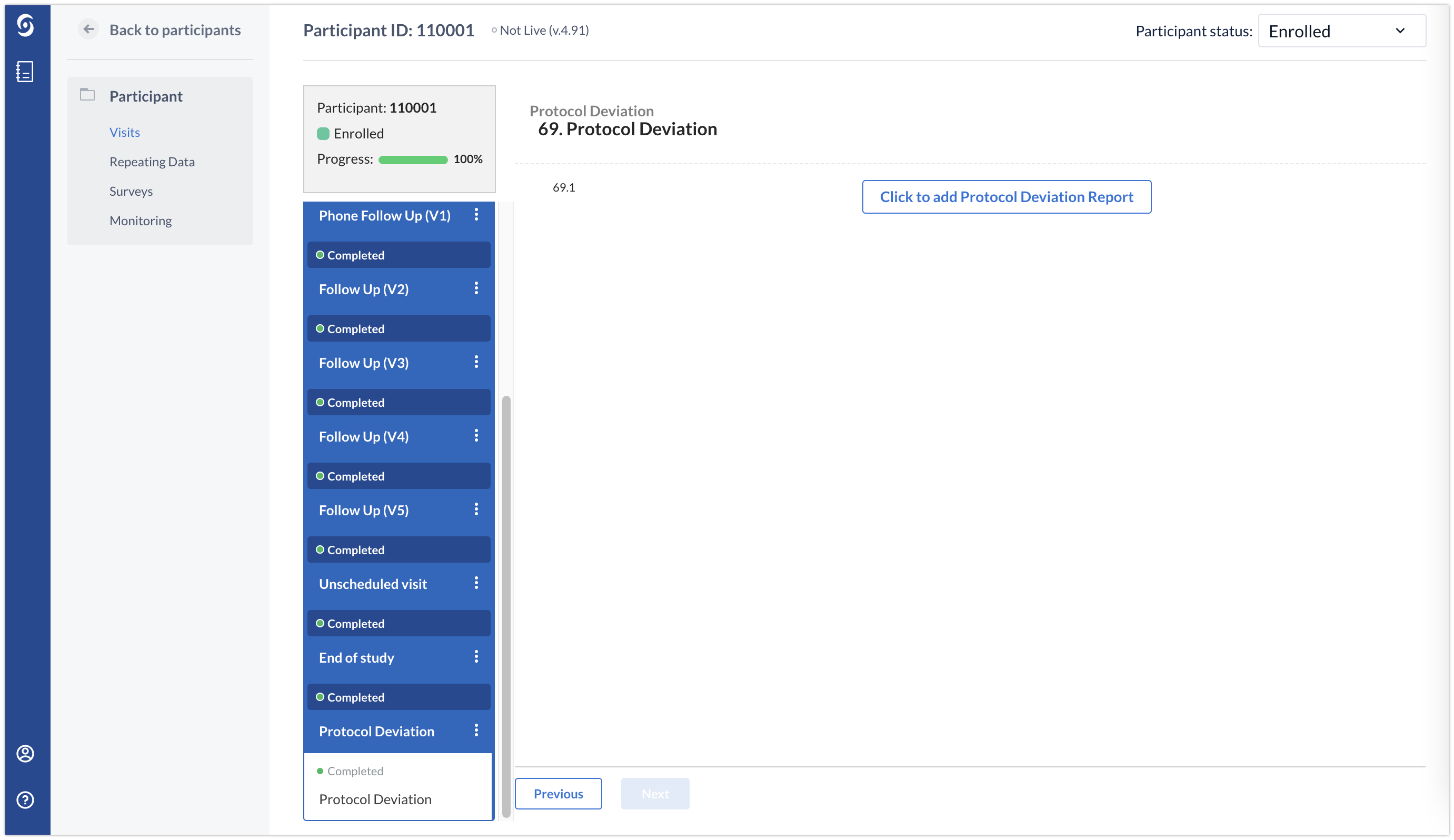
Using the ‘Repeating Data’ button, the Protocol Deviation repeating data instance will always be linked to the visit in which the repeating data instance was created.
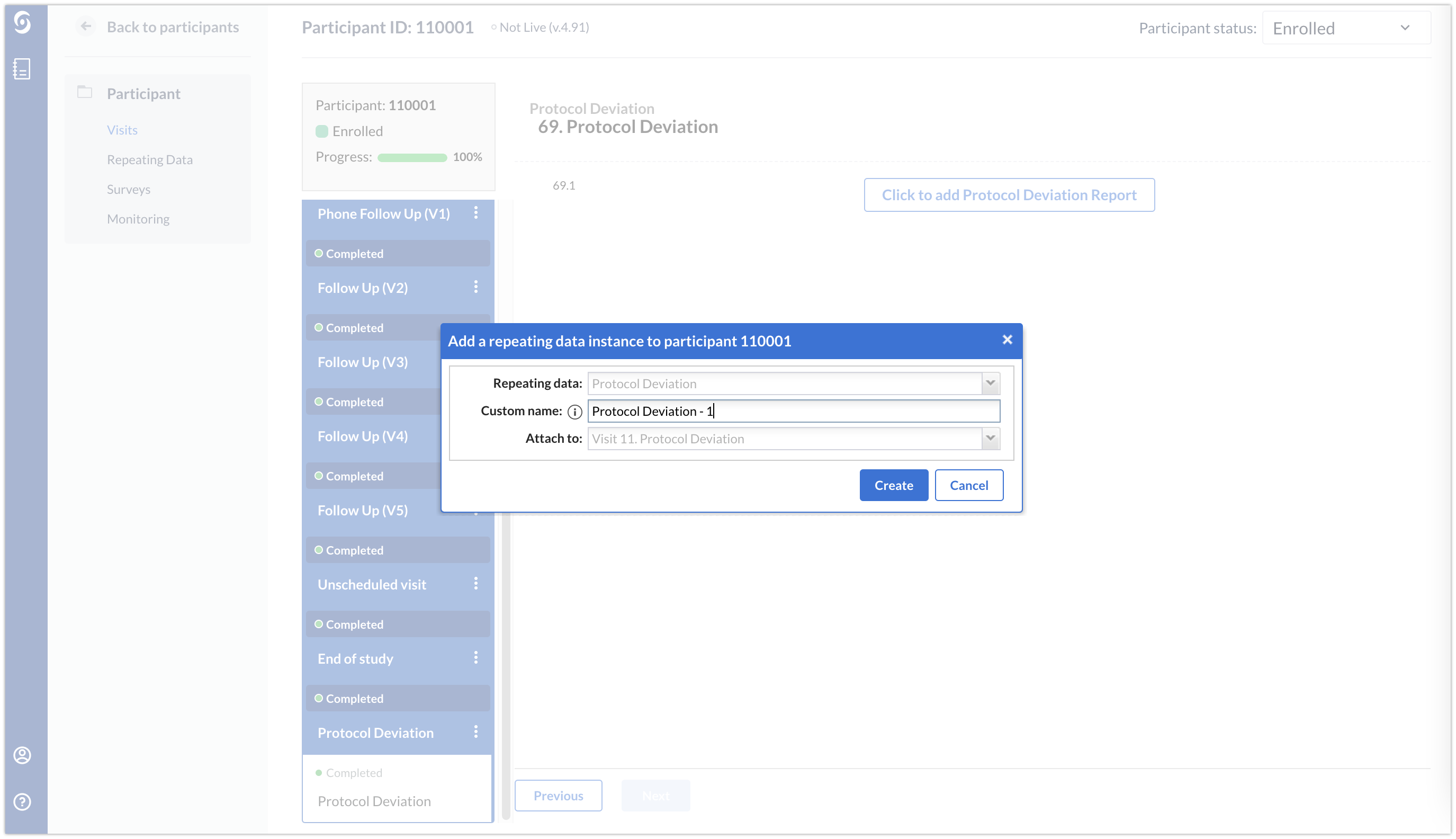
Check if a deviation was added
You can check to see if a deviation was added to a report by either of the following methods:
- Check if a repeating data instance was added
- Creating Notifications on the Repeating Data Instance Event type and choosing the Deviation Report.