Create a new participant in CDMS
To create your first participant, you must have at least one form created. To start adding fields to your forms, it is necessary to create a visit and form in the study structure.
To create a new participant, follow the instructions provided below:
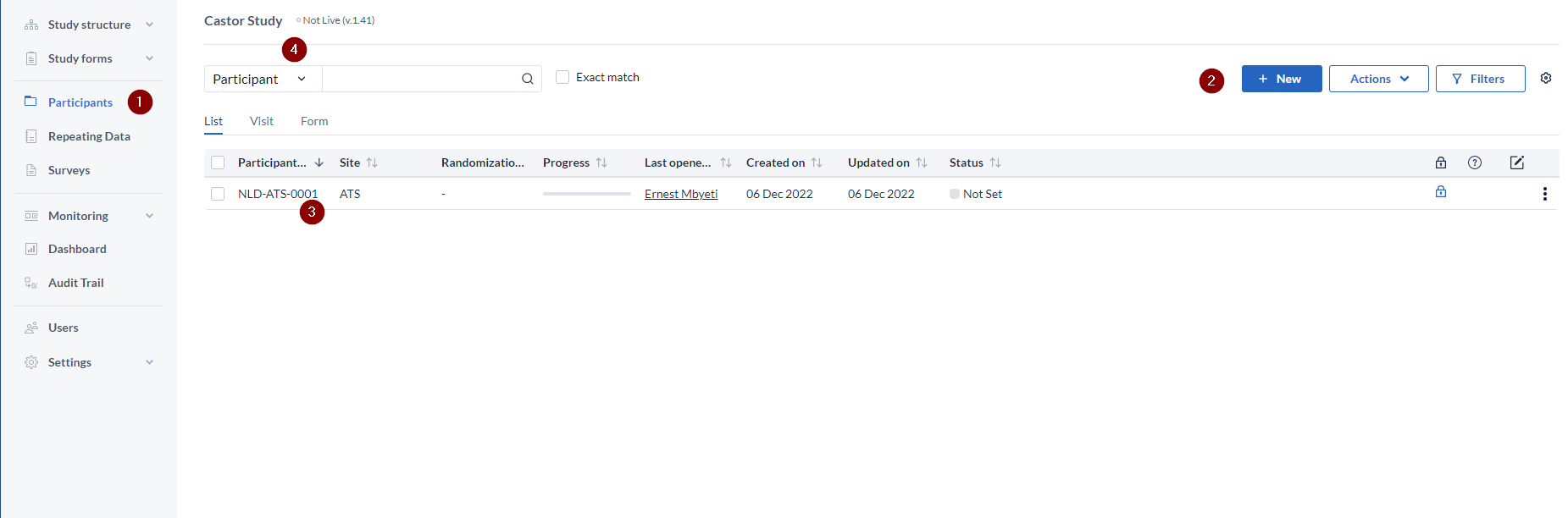
- Click on the Participants (1) page
- Click on + New button (2). You will be prompted to select the site to which the participant is linked.
If you can't find your site listed, you can follow these instructions to add it to your study.
By default, Castor CRF will generate incremental IDs per site. There are however other ways to set up custom IDs. See Customize participant IDs for more details.
Users with permissions to create participants can directly enter the participant's email address and associate it with that upon the creation of the participants.
- Once you click on Create, your participant will be created and will appear in the 'Participants' tab overview.
- Enable the option Create another to proceed with creating another participant right away.
- After creating your participant, you can search for participant in the search bar.
- When creating a participant with the ‘Create another’ option disabled, you will be immediately forwarded to the data entry view for this participant.
Please note that when creating a new participant with the participant ID generation being set to ‘Patient Study ID (free text) from the settings tab, adding the characters ‘[ARCHIVED-]’ in the field is not allowed to prevent possible errors upon archiving participants, as this prefix assigned automatically upon archiving participants.