Track participant progress in CDMS
Table of Contents
Discover how to easily record and monitor individual participant progress across clinical trials and studies in Castor CDMS.
The Participants tab
- Navigate to the 'Participants' tab. Here, you have an overview of all of the participants in your study. You will automatically be taken to the 'List' view mode.
- There are two other views in which you can find more detailed information about the progress of your participants: the 'Visit' view and the 'Form' view.
- Each participant has a progress bar that indicates the completion status of the participant.
Progress bars are color-coded:- Grey/empty bar: data entry has not been started for this participant
- Partially blue bar: data entry is in progress
- Full green bar: all fields are completed for the participant
- Full purple bar: an exclusion message has been triggered for this participant
Progress by visits
In the 'Visit' view mode you can see the status of each visit for each participant:
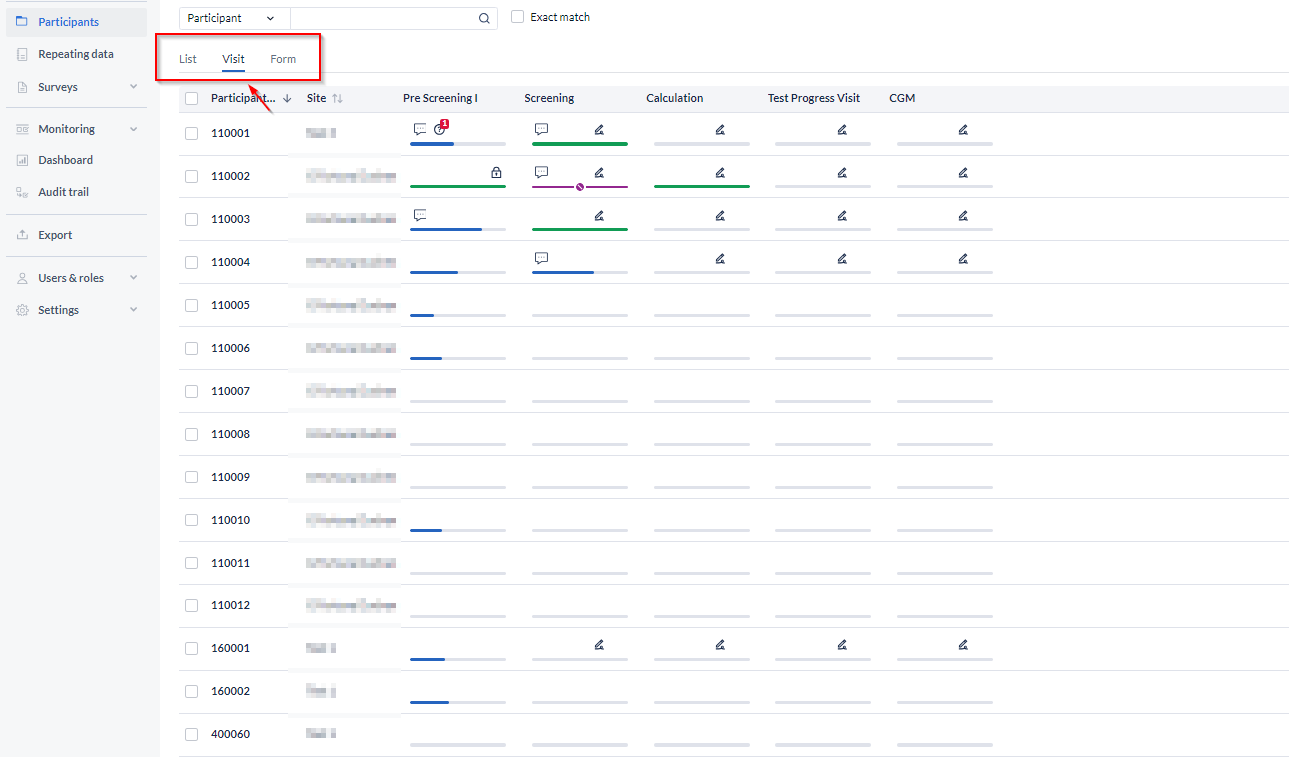
Clicking a cell will open the participant on the selected visit, so you can quickly navigate to visits requiring your attention. The visit icons are color-coded:
- Green - visit completed;
- Blue - visit started but incomplete;
- Grey - visit not started yet.
Additionally, each visit can have small icons noting that the visit is locked, signed, or has an open query.
Progress by forms
The 'Form' view follows the same principles as the 'Visit' overview. Here you can see the status of each Form for each participant.
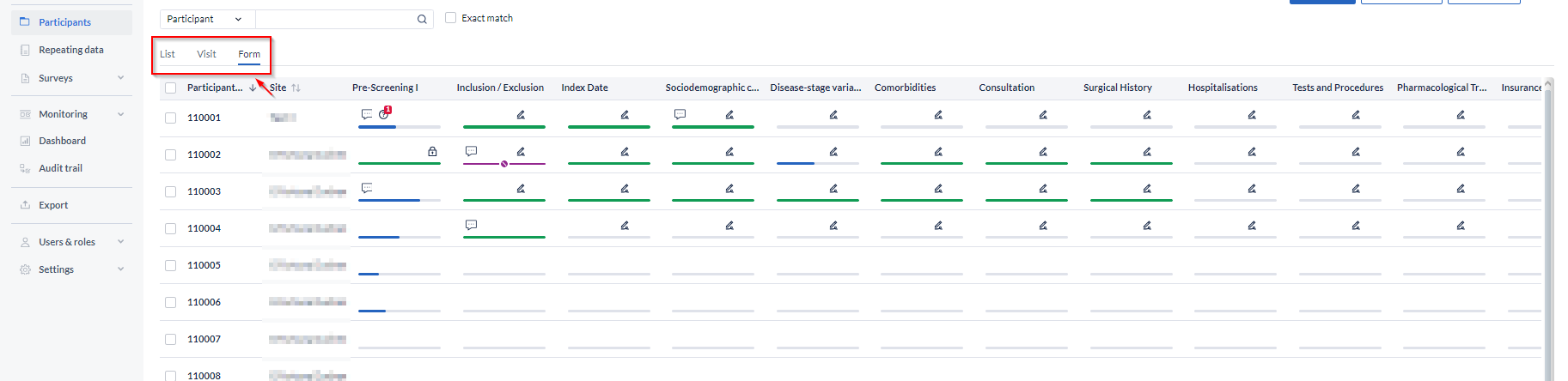
Double-clicking a cell will open the participant on the selected form, so you can quickly navigate to forms requiring your attention. The form icons are color-coded in the same way as the visits.
Statuses of visits and forms
Additionally, each visit and form can have small icons indicating the following statuses:
- a lock icon indicates a locked visit or form.
- a pencil indicates a signed visit or form.
- a question mark icon indicates the presence of a query that is not Closed.
- an SDV mark indicates a verified form.
- a comment icon indicates the presence of one or multiple comments.
- a crossed-out eye icon indicates a User Role is not allowed to see the visit.