Parameters used in the API in EDC/CDMS
Table of Contents
The Castor CDMS API Documentation page gathers the methods and requirements of the fields in Castor to be used with an API.
The Changelog of the Castor CDMS API can be reviewed here.
There are a handful of parameters that are used to locate the data in the database and retrieve it or update it. See the table below:
| Parameter |
Description |
|---|---|
| {user_id} | Numeric identifier of the user (UUID) |
| {country_id} | Numeric identifier of the country (use the GET method for a list of IDs) |
| {study_id} | ID of the study (found in Settings > Study) |
| {field_id} | Identifier of the field |
| {record_id} | RecordID as shown in the Participants tab |
| {report_id} | Identifier of a repeating data (structure) |
| {report_instance_id} | Identifier of a repeating data instance added for a Record |
| {survey_id} | Identifier of a survey (structure) |
| {survey_instance_id} | Identifier of a survey added for a Record |
| {survey_package_id} | Identifier of a survey package (structure) |
| {survey_package_instance_id} | Identifier of a survey package added for a Participant |
| {survey_step_id} | Identifier of a survey form |
What is the difference between ID and instance ID?
Each component of the study is identified by a unique ID: field, user, etc. Some of them are simply referenced by "ID", like the studyID and the recordID. In the user interface these items locate in the Study structure and Study forms tabs:
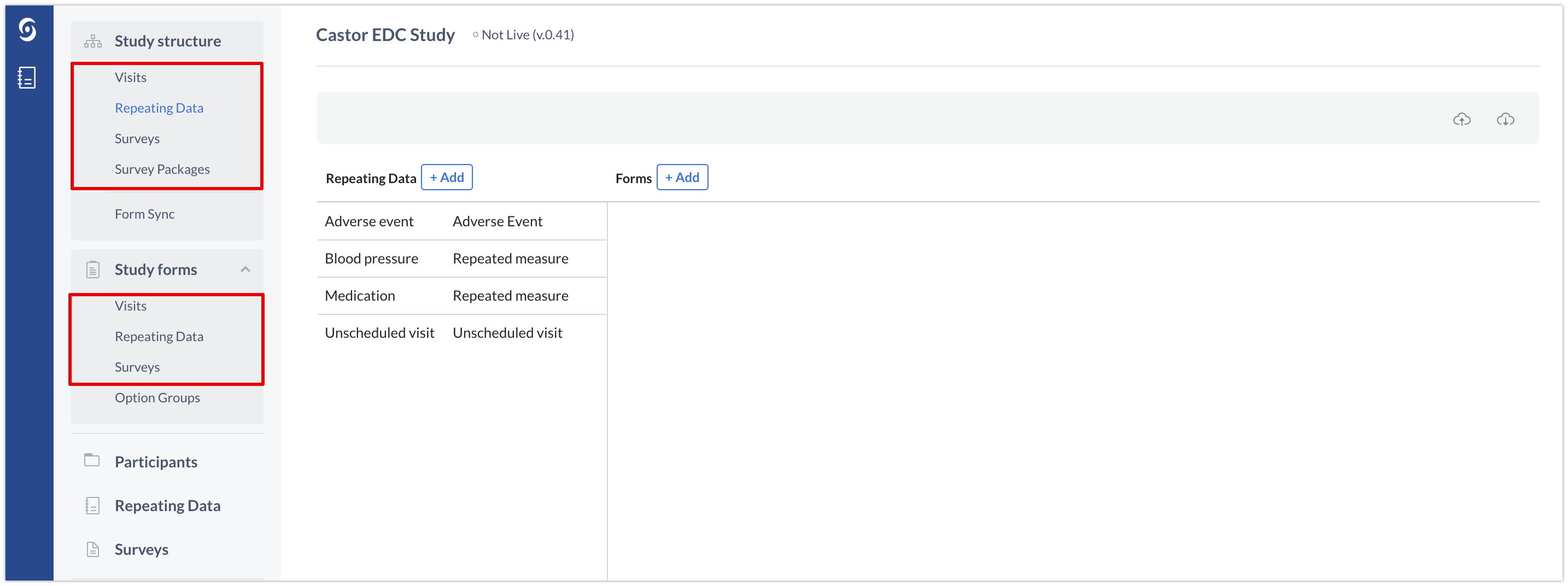
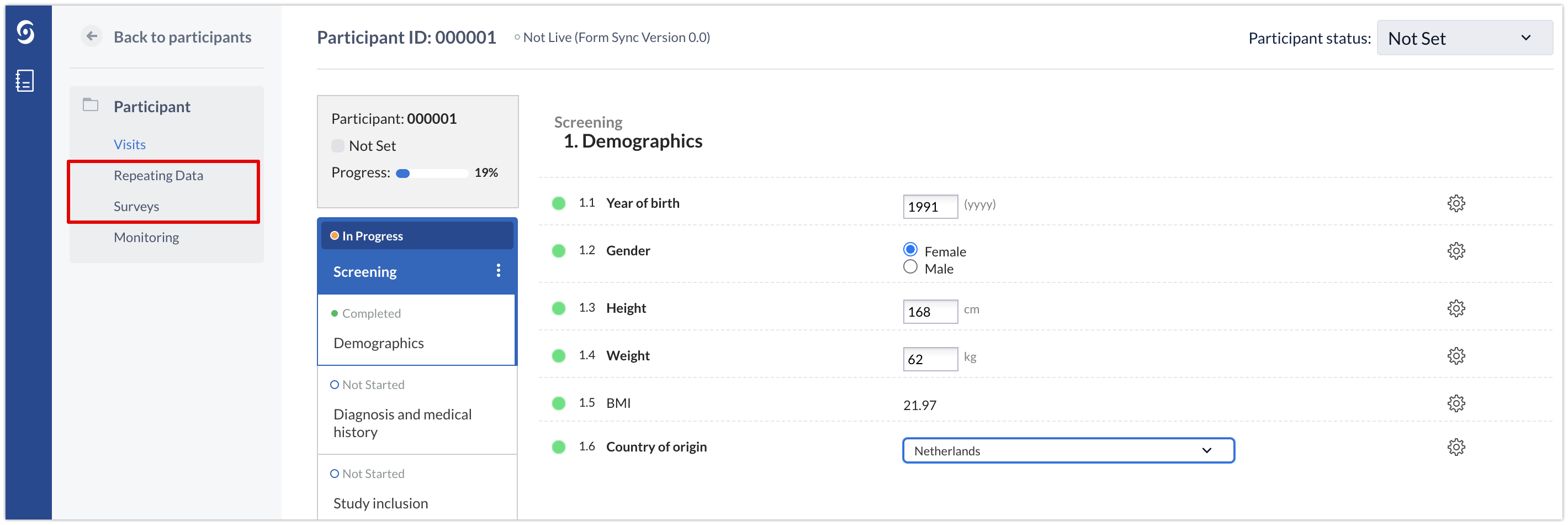
However, some components can appear repeatedly in the study even within the same participant. Such is the case of repeating data instances, surveys and survey packages. For this reason we need a unique ID to identify each of the occurrences of these components across the study. This unique identifier is the instanceID.
These items that are referenced with an "instanceID" are always located in the menu inside each participant, in the Participants tab of the user interface:
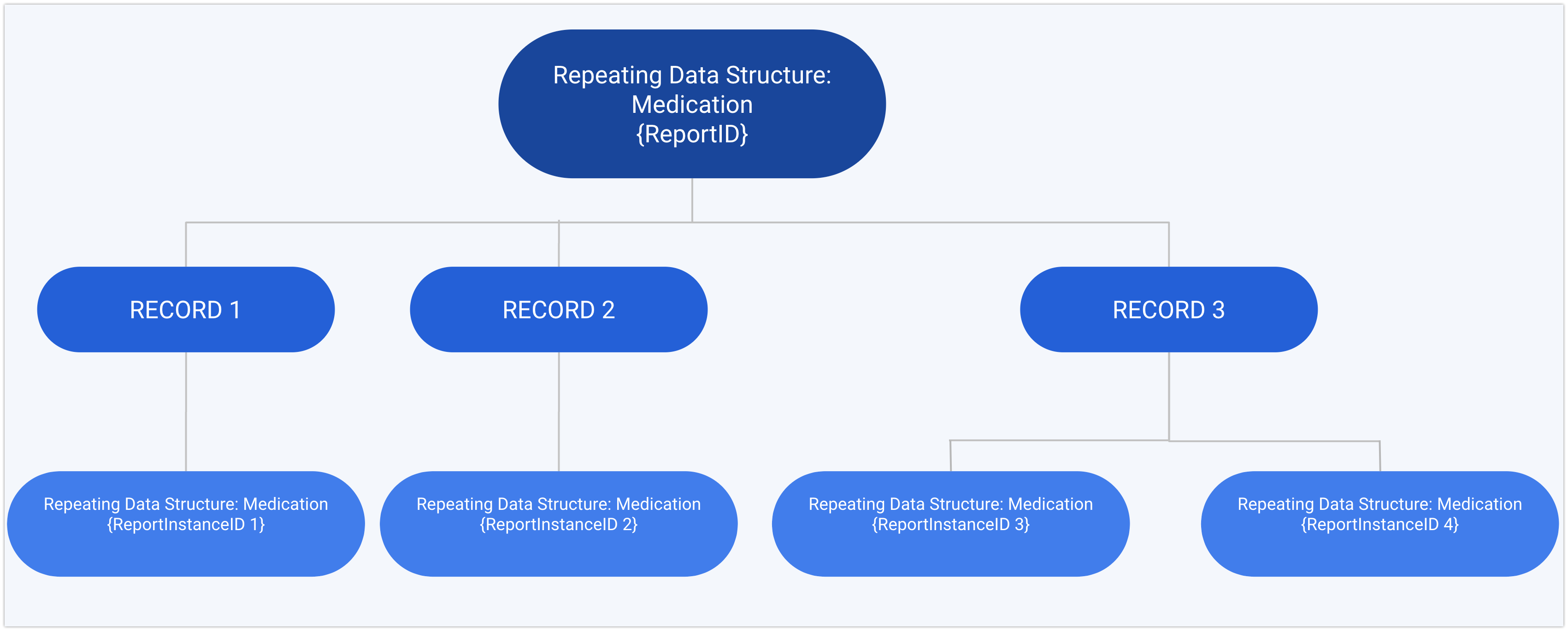
The following chart shows an example for the relation between ID and instance ID for a repeating data structure called "Medication":
See also Where can I find IDs for the study/field/repeating data instance/... for the API? article to learn how to find the IDs for the API, and The structure of Castor for a comprehensive description of the parts of a study.