Why can't I open/enter my study?
It is possible that you're trying to open a study and that you see the following error message:
'Could not open selected study. You don't have permission to enter this study. Please contact your study admin to request permission.'
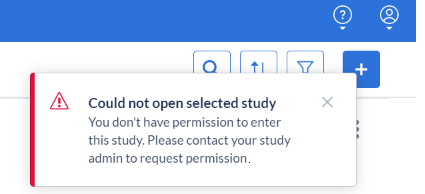
This means that you do not have sufficient user rights to enter the study while it is not live. Users will need at least one management right to access a study that is not live.
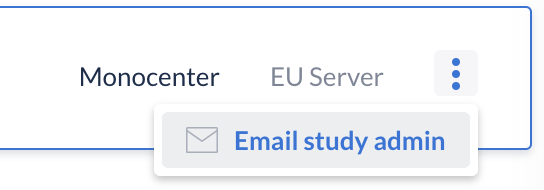
Please contact your study admin, if you receive this message and you think you should have access to the study. The study admin can set the study live again, or give you the appropriate user rights. You can contact them by clicking on the three dots on the right side of the study, then click on 'Email study admin':