How can I add new sites in EDC/CDMS?
Table of Contents
When setting up a multicenter study, new sites can be added via the 'Settings' tab in the study.
To add a new site, follow the steps below:
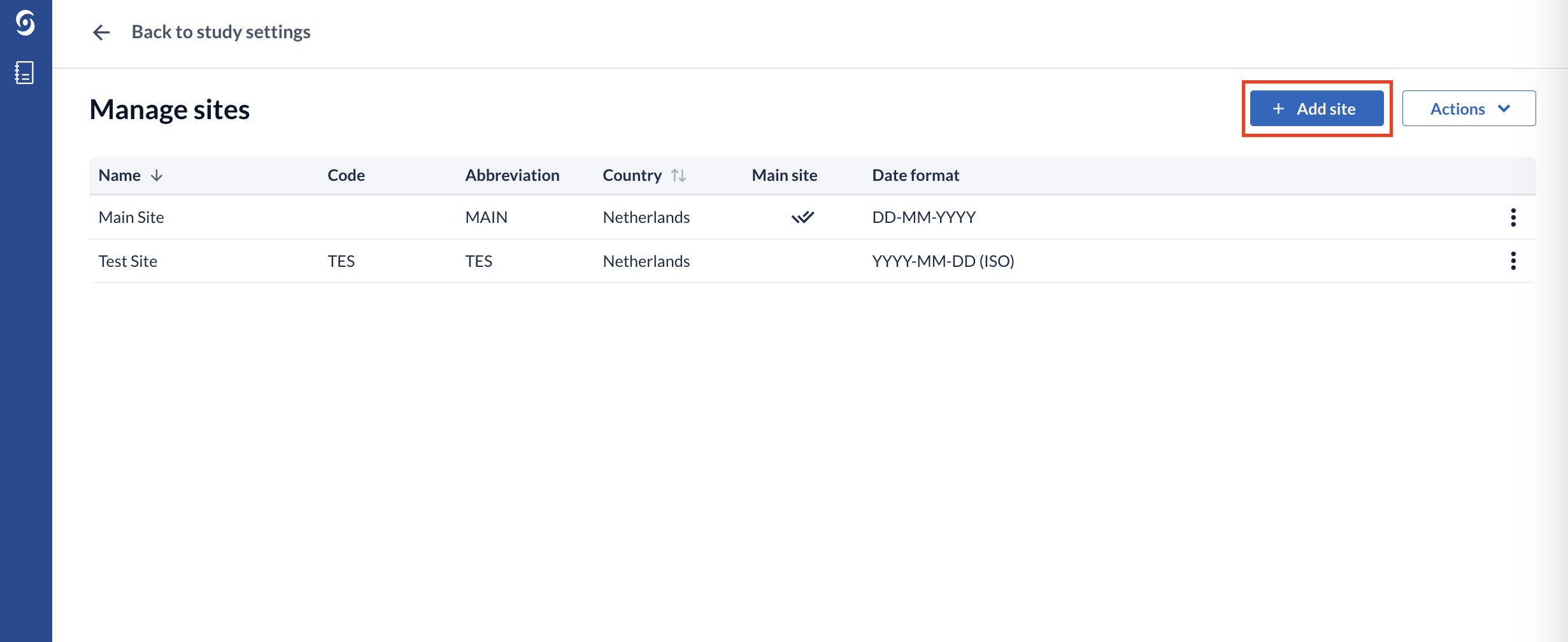
1. Click on the 'Manage sites' button. A a page ‘Manage sites’ will be opened, which contains a list of all existing sites:
2. New sites can be created using the 'Add site' button.
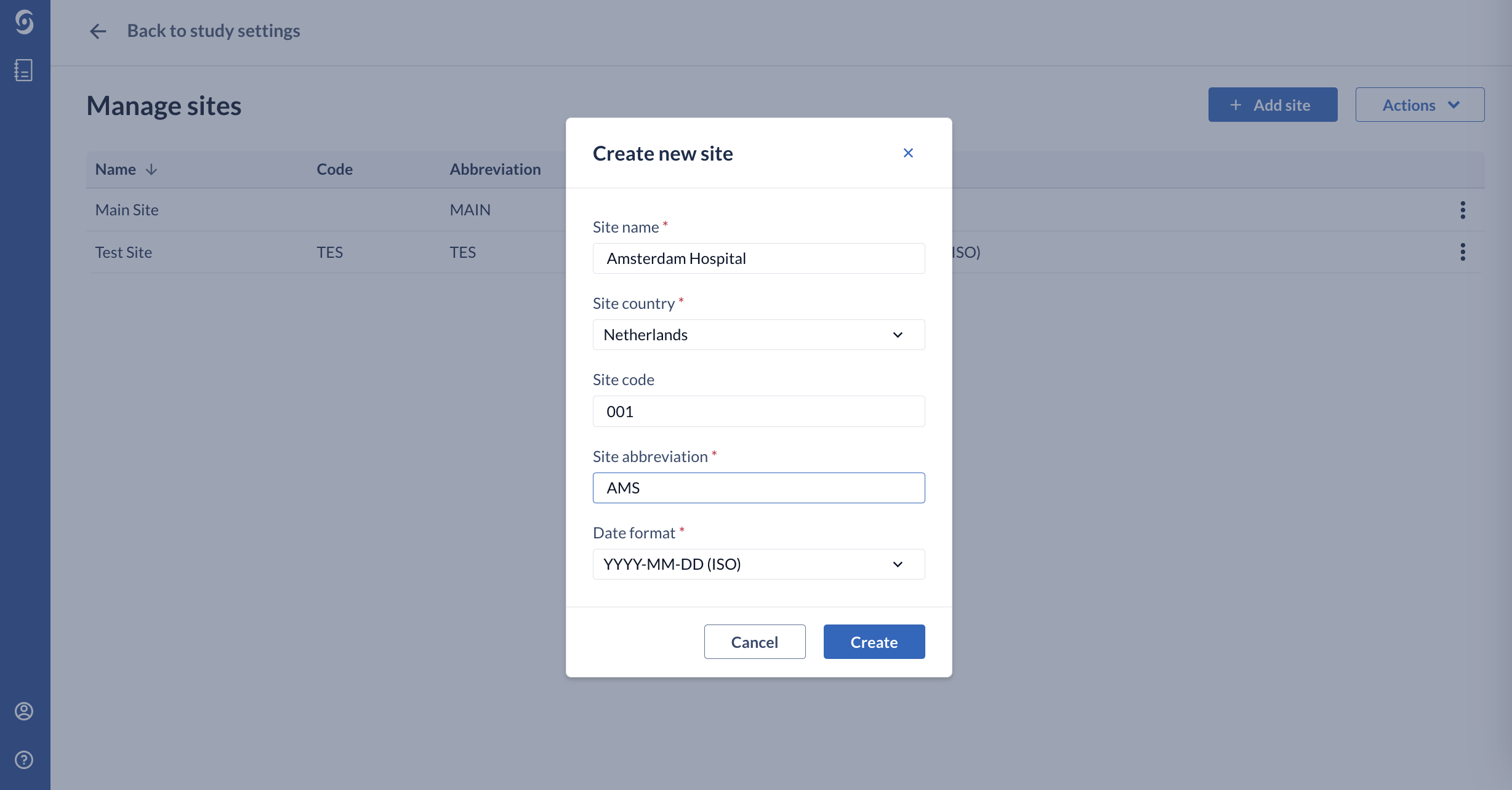
3. In the dialog window ‘Create new site’, you should provide the name of the site, the country and select an abbreviation which can be used within the study. This information can be changed at any time.
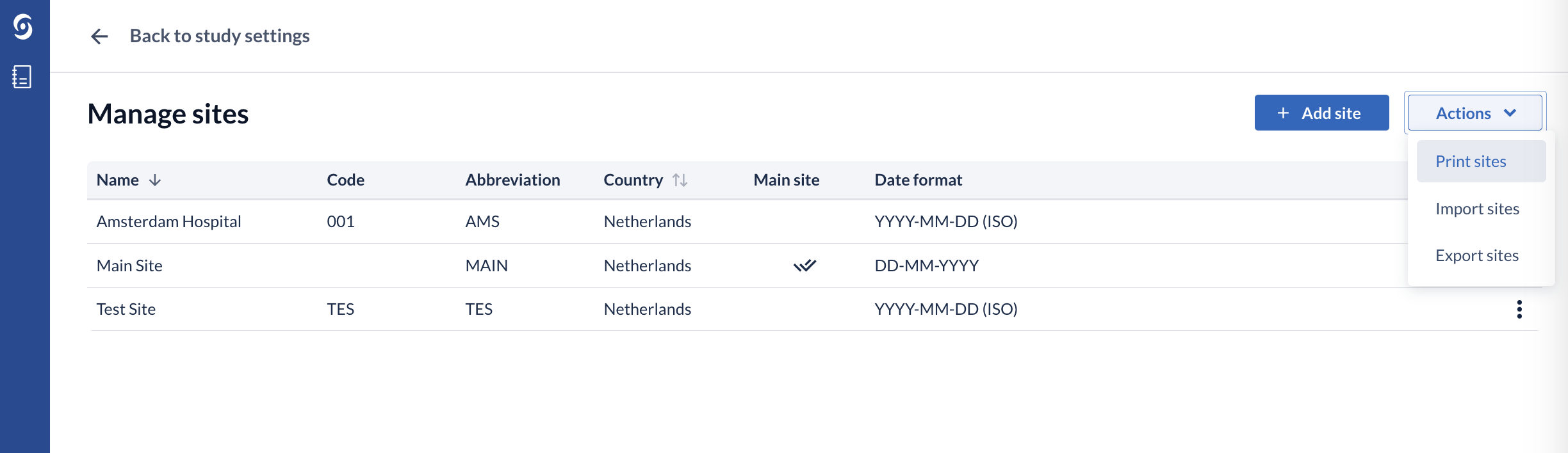
Once a new site is created, you should update the User rights to allocate rights for the new site. No rights are given automatically for new sites, so it will not be possible to add new participants to the new site until rights are updated.
You can view and print an overview of all sites and all user roles and permissions per site using the 'Print sites' option in the ‘Actions’ menu.
Editing or Deleting a site
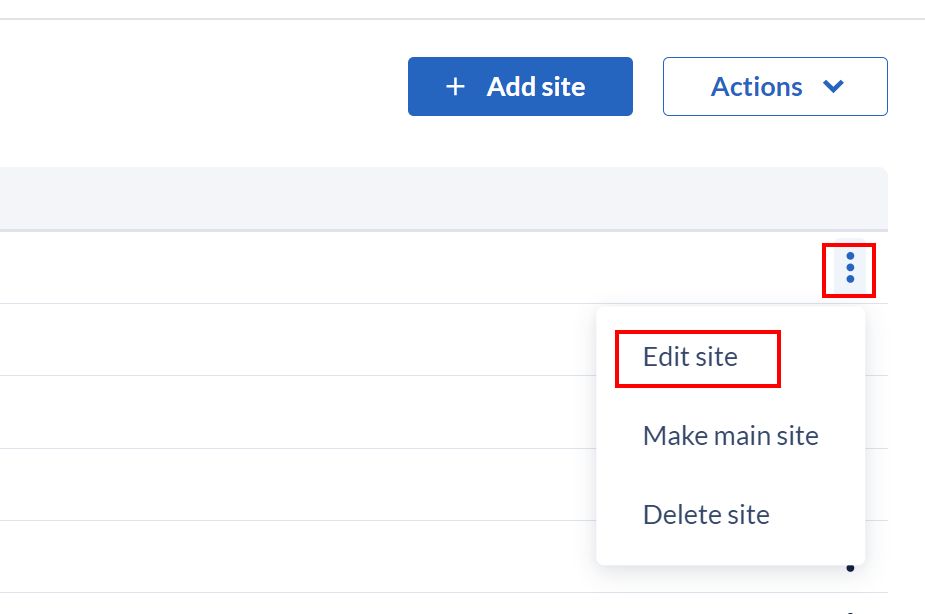
Clicking on the context menu icon (three dots) to the right of the site opens a drop down list where you can edit, make the site the main site, or delete the site.
To edit a site, select ‘edit site’ and a dialog window showing the properties of the existing site (Name, Country, Code, Abbreviation and Date format) will appear. Here you can update the site properties as needed.
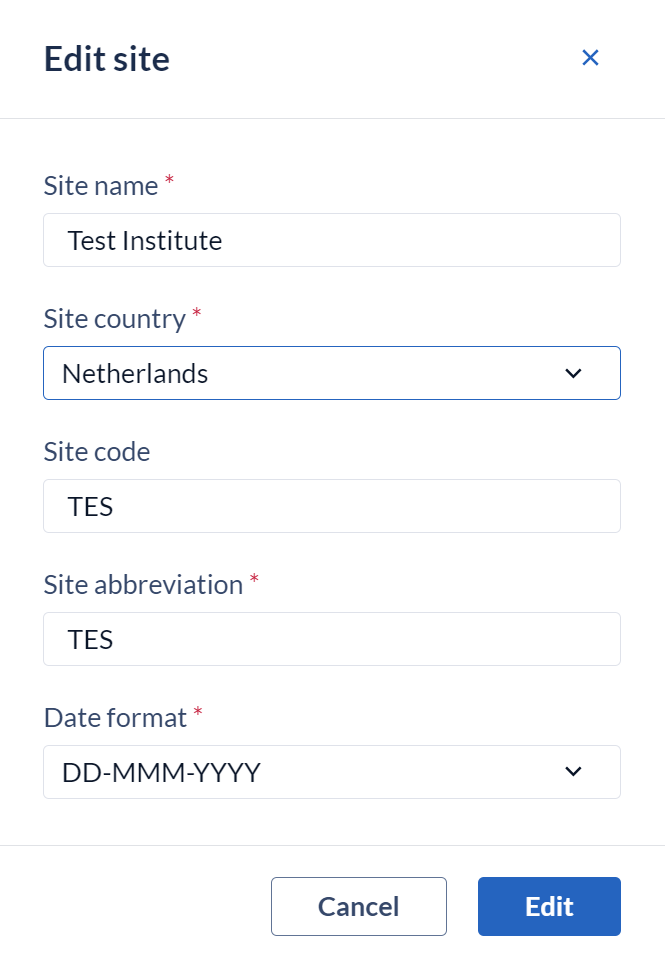
Once all changes have been made select ‘Edit’ to save the changes.
To delete a site, click the context menu icon (three dots) and choose the option to ‘Delete site’. Please note that if the study is currently or has previously been 'live' and there are participants in the study associated with this site, it cannot be deleted.
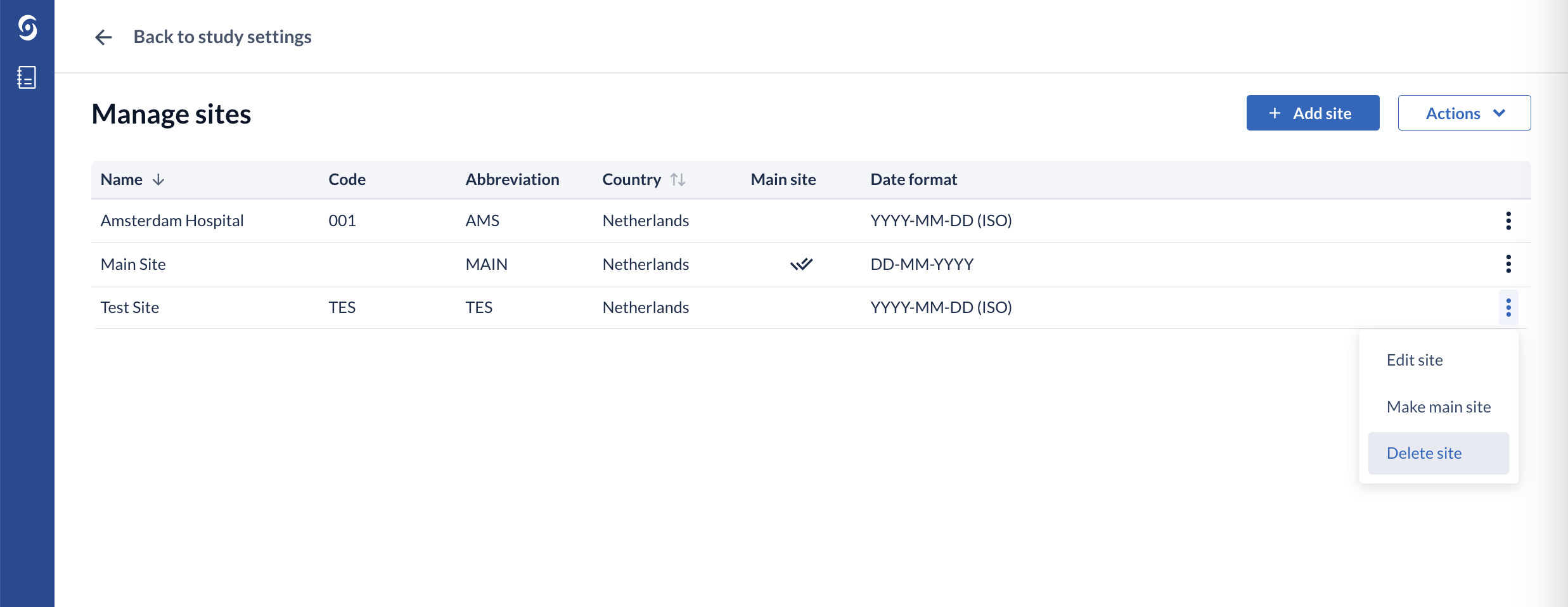
Select the ‘Make main site’ option to change the main site. A pop-up window will appear to confirm the change of the main site.
Use the Import sites functionality to add sites in bulk: Import sites in CDMS