Define custom date format per site in CDMS
tabFlexible date formats gives users the possibility to define, per each available study site, the preferred date format to be used and displayed within data entry.
To define custom date format, follow the steps below:
1. Navigate to the Settings → Study sub-tab → navigate to the ‘General’ section
2. Click on the ‘Manage sites’ button. On the ‘Manage sites’ page, you can view and choose to update the selected date format.
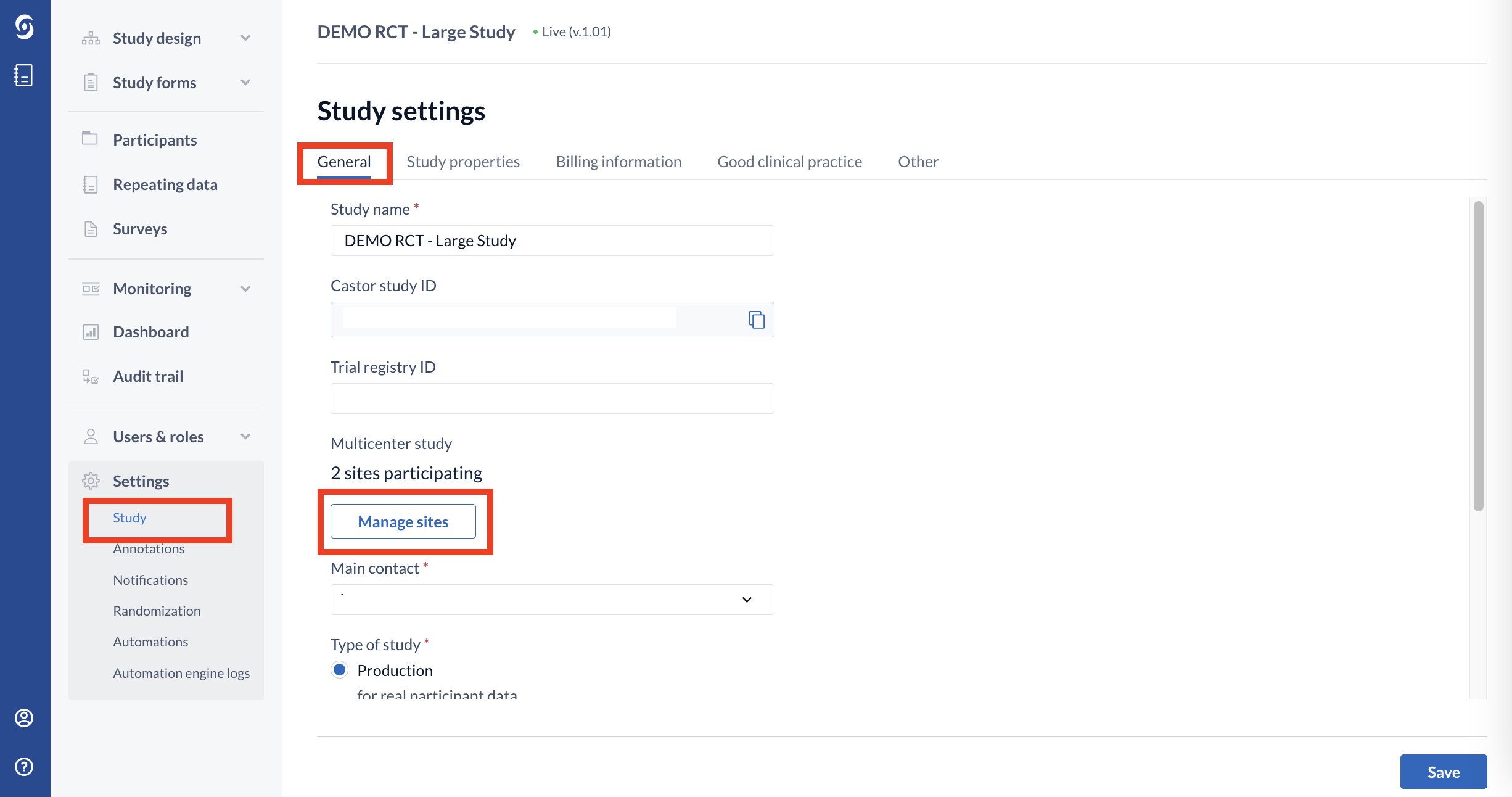
3. Click on the context menu (three dots) icon next to a site where the date format should be customized → click ‘Edit site’ option:

4. Choose the desired data format in the ‘Date format’ dropdown menu and click ‘Edit’ to save the changes:
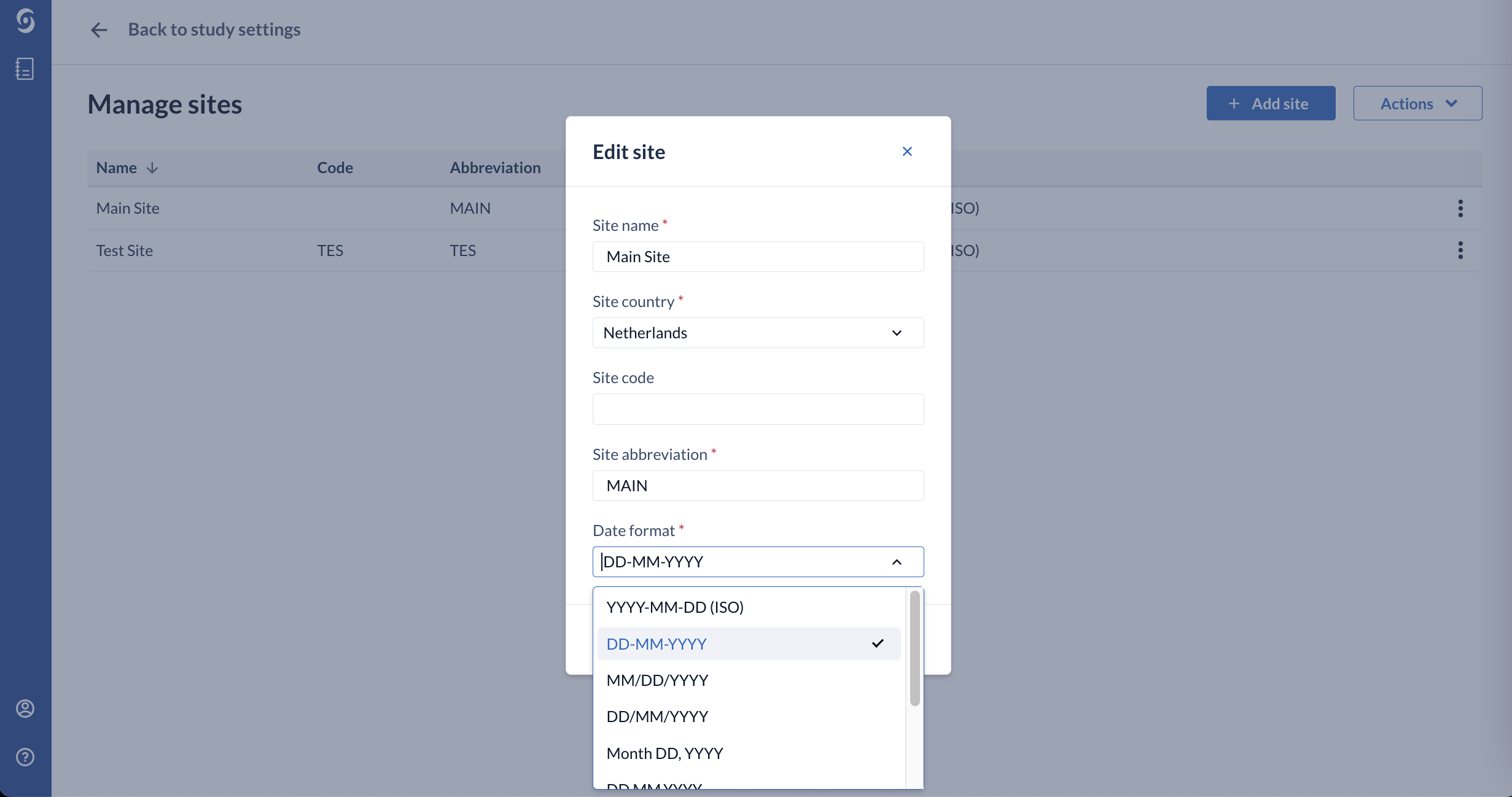
When creating a new site, you can select the desired date format from the ‘Date format’ field as well.
A list of nine (9) different formats is available, from which you can select one for each site:
- YYYY-MM-DD (ISO)
- DD-MM-YYYY
- MM/DD/YYYY
- DD/MM/YYYY
- Month, DD, YYYY
- DD Month YYYY
- DD-MMM-YYYY
- YYYYMMMDD
For all studies created after the release of the 2021.5 version on 11 October 2021, the default date format will be the standard ISO format (YYYY-MM-DD), while for running studies the default will remain with the previous format that was available before EDC version 2021.5.
Depending on the site date format, the view in data entry for each participant will reflect that on all relevant field types: Date, Date and Time, Date and Number.
Dates are currently stored internally as DD-MM-YYYY and will also be displayed as DD-MM-YYYY when exported.