Customize participant's ID's in CDMS
Table of Contents
This article covers how to change the way participant IDs are generated within Castor CDMS.
Participant ID Options
First, navigate to ‘Settings’ → ‘Study’ tab → ‘Other’.
Click the ‘Participant ID’ dropdown to see the various options for customized participant ID's:
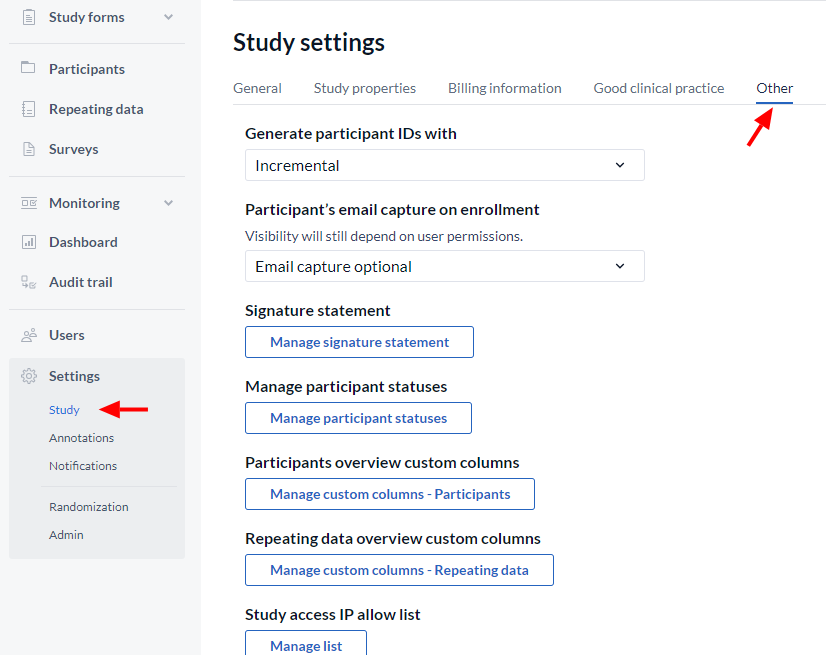
Below are the options for customizing your participants IDs:
Random number: This will create a random number for each new participant.
Patient Study ID (free text): Use this option if you want IDs to be entered by users when creating participants.
Note: We advise you to only use if participant creation is being handled by an API and not to use hospital IDs
Incremental / Incremental per site: This option will create incremental IDs for each participant per site. The participants for the first site will be numbered: 110001, 110002 etc, the participants for the second site will be numbered 120001, 120002 etc. (The numbering starts at '11', because 10001 and 100001 are really hard to distinguish).
Incremental per country / site code / site abbreviation: Similar to the option above, but will generate participant IDs with country and custom site codes (i.e. NL-AMC-001, NL-VUMC-001, etc).
Custom definition: You can also create your own participant ID generation pattern, as described below.
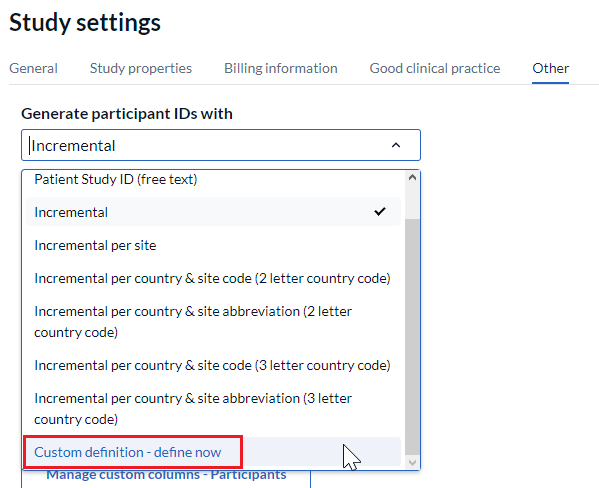
The new pattern will only be applied to the newly created participants. If you would like to change the ID for existing participants, follow the instructions here.
Custom Participant ID Generation Patterns
When you choose the option to create a custom definition you will see this menu:
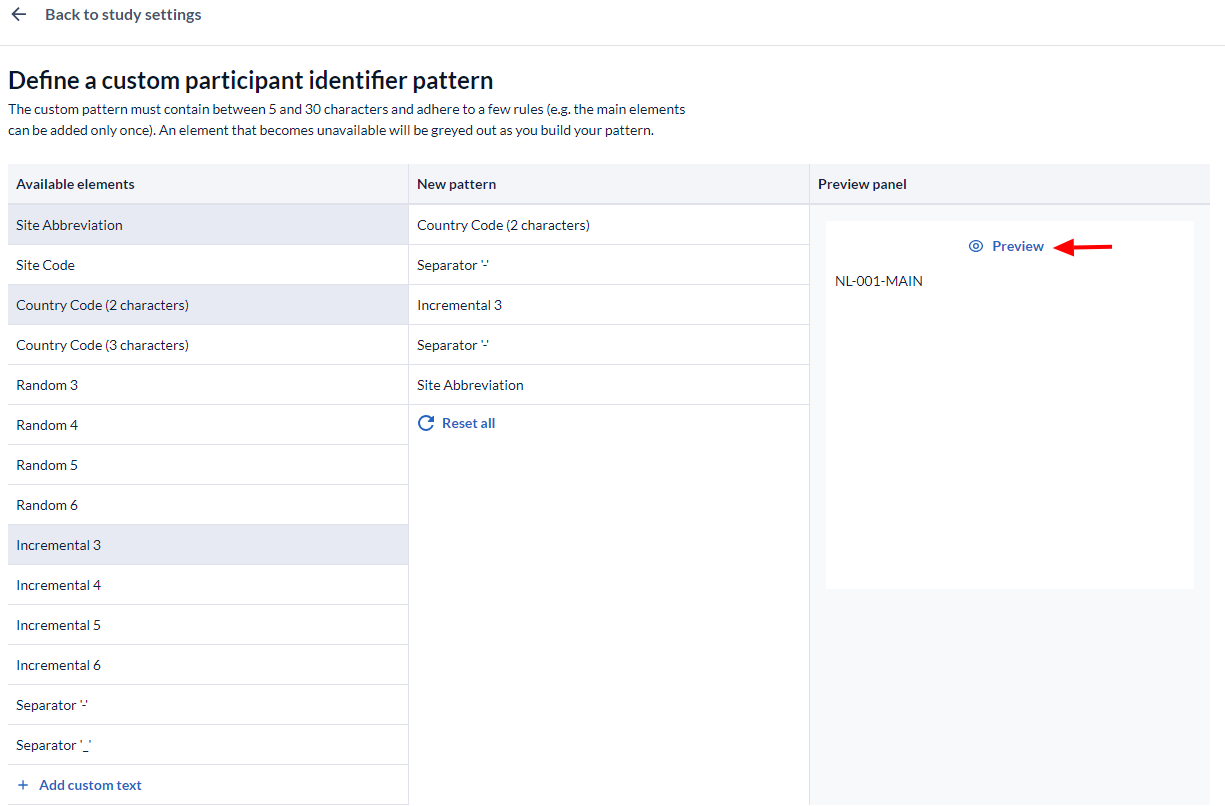
With the custom generation patterns you have a lot of control over your participant IDs.
- Sequentially select elements from the "Available Elements" list on the left to create a new pattern.
- The selected elements will be shown in the 'New Pattern' panel, click the X to remove any unwanted elements.
- Press the Preview button to see how your pattern would look with the site/Country information you supplied earlier in the Manage sites dialog.
- This box shows a preview of how your participant IDs should look.
Press ‘Save & Use’ to add the custom pattern you created for the generation of participant ID's to your study.
The incremental part checks for anything before the incremental number. Thus, adding the incremental at the beginning of the ID will result in a different sequence to adding it in the middle or at the end. It is recommended to test the options extensively to prevent issues at a later time point.