Notifications for study events in CDMS
Table of Contents
Castor CDMS can send you updates via email (notifications) of several predefined events as soon as they happen in the system. This article explains how to set up such notifications about certain events in your study.
| You can also learn more about notifications within Castor Academy. Click here to view the Castor Academy ‘EDC-CDMS - Notifications (v03.2025)" course. |
Setting up Notification
To set up notifications, first go to the study 'Settings' tab, then choose the 'Notifications' sub-tab. Here you can add a new notification or edit existing ones. When clicking the 'Add notification' button or the edit cogwheel, this menu will appear:
When adding a new notification there are several options to be set.
-
Event type: Choose one of the currently available events:
- Field result: Repeating Data
- Field result: Study
- Field result: Survey
- Form signature dropped due to edit
- Form signed: Repeating Data
- Form signed: Repeating Data Form
- Form signed: Study Phase
- Form signed: Study Form
- Form verification dropped due to edit (and field form verification dropped due to edit)
- New query created
- New participant created
- Query updated
- Participant randomized
- Repeating data instance completed
- Survey package completed
- New repeating data instance added to participant: when selecting new repeating data added to a participant, choose from the drop-down menu which is the specific repeating data that you are interested in receiving the notification
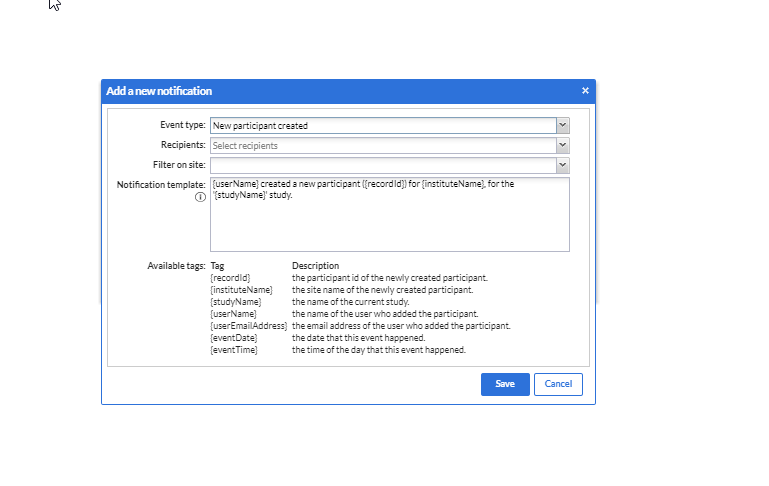
- Recipient: Choose a recipient of the notification email in the drop-down (which shows all users added to the study). The 'Recipient' dropdown allows for multi-select which makes it possible to select and deselect all study users. Email notifications are sent out to all selected recipients individually.
- Filter on site: Choose one or multiple sites for which you want to receive the notifications (i.e. only your own hospital). Leave this field empty if you want to receive notifications for all sites.
- Notification template: This is the email text that will be sent when the event occurs. You can modify this as you like. The listed available tags will be replaced by their real values when the notification is sent. You can find an overview of all available tags for each study event in the document: Notification tags
Press the 'Save' button to save the notification or the cancel button to return to the notifications overview.
Field level notifications
For the event ‘Field result’, three notifications can be created, each one corresponding to either study, repeating data, or survey fields (data point).
One minute after filling the configured field, the selected recipient will receive a notification about the action. The notification will be triggered in the following events:
- when the data is changed or added to a field manually and no email will be sent when the data is imported,
- when the field result is updated, for example (new data added, data is changed, field is cleared). At the moment it is not possible to trigger a notification for a specific field result.
Notifications are not supported for the following field types:
- Remark
- QR code
- Image
- Link
- Summary
- Calculation
- Add repeating data button
- Add survey button
- Upload file field