Study management tabs in in CDMS
Table of Contents
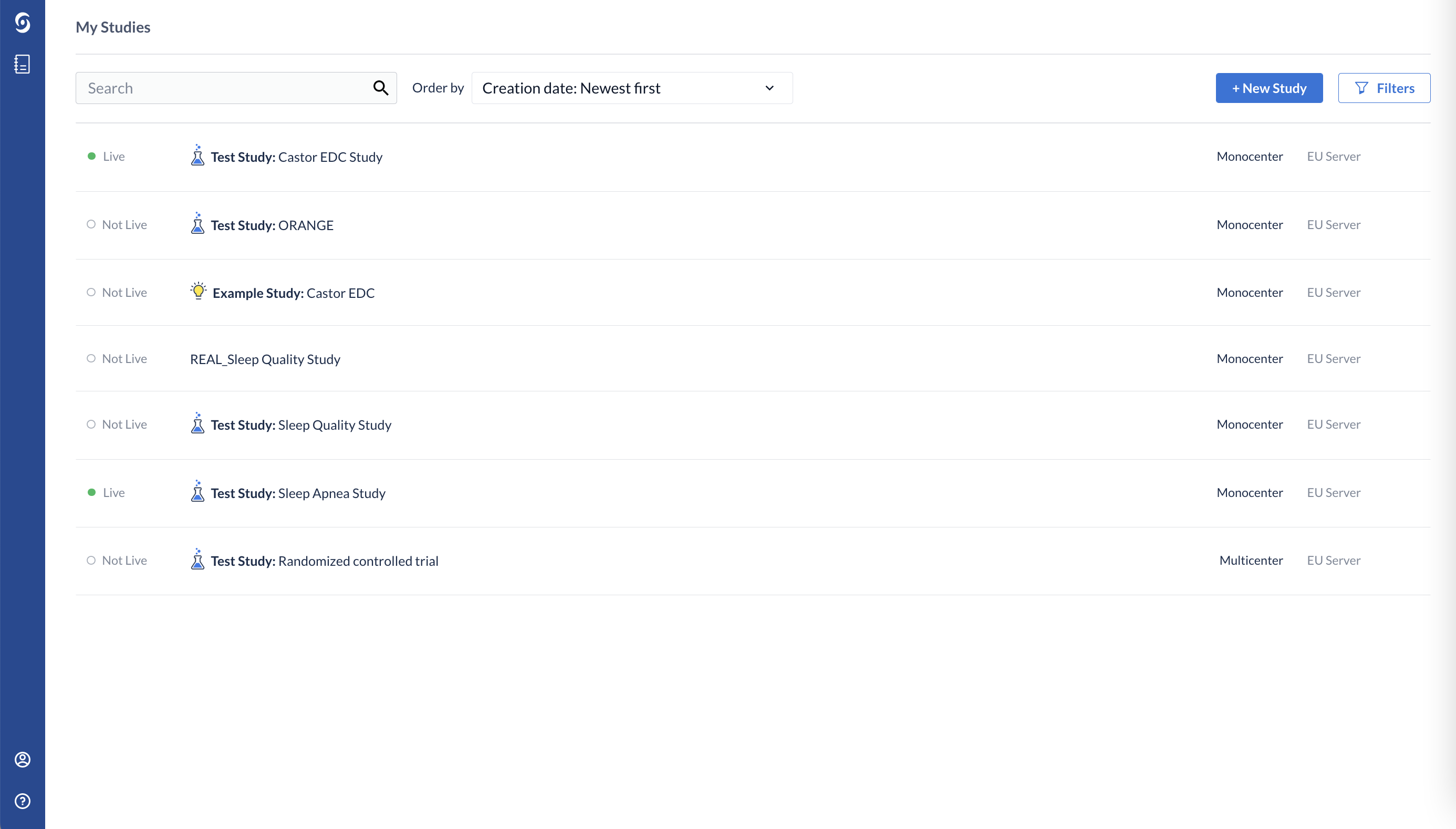
After logging into the CDMS, you will be redirected to the ‘My Studies’ list. Here you will see the list of all the studies you have created or been invited to.
Open a study for management
To open your study for management you need to have at least one of the ‘Manage’ rights.
On the ‘My Studies’ page click on the study row to access a study:
The 10 management tabs
There can be up to ten tabs visible in the study manager's view, depending on the rights you are allocated in the study.
- Participants: From this tab, participants can be accessed without the CRF being 'live', this allows study forms to be tested during study development. By default, this will be the first tab you will see when opening your study.
- Study Structure: This is where you define the structure of your study. This is the first step you take when building your study.
- Study forms: Here the Form Builder is displayed. This is where the CRF forms are built.
- Repeating Data: Here is an overview of all repeating data added to the study.
- Surveys: Here you can manage all the survey invitations that have been created.
- Monitoring: Only visible when using the additional GCP package, the Monitoring tab shows an overview of all queries, verifications and validations for the current CRF.
- Statistics: This tab shows number of inclusions and the number of participants randomized per site per group.
- Audit Trail: An overview of the complete audit trail for the study. This tab will only be visible when you have all management rights enabled for your user (i.e. Manage participants, Forms, Users, and Settings).
- Users: Here users can be invited to a CRF, and rights for each individual user can be managed.
- Settings: Here all the settings for the eCRF are managed.
Go back to the 'My studies' page
You can go back to the 'My studies' page with the overview of all your studies by clicking on the Castor icon: