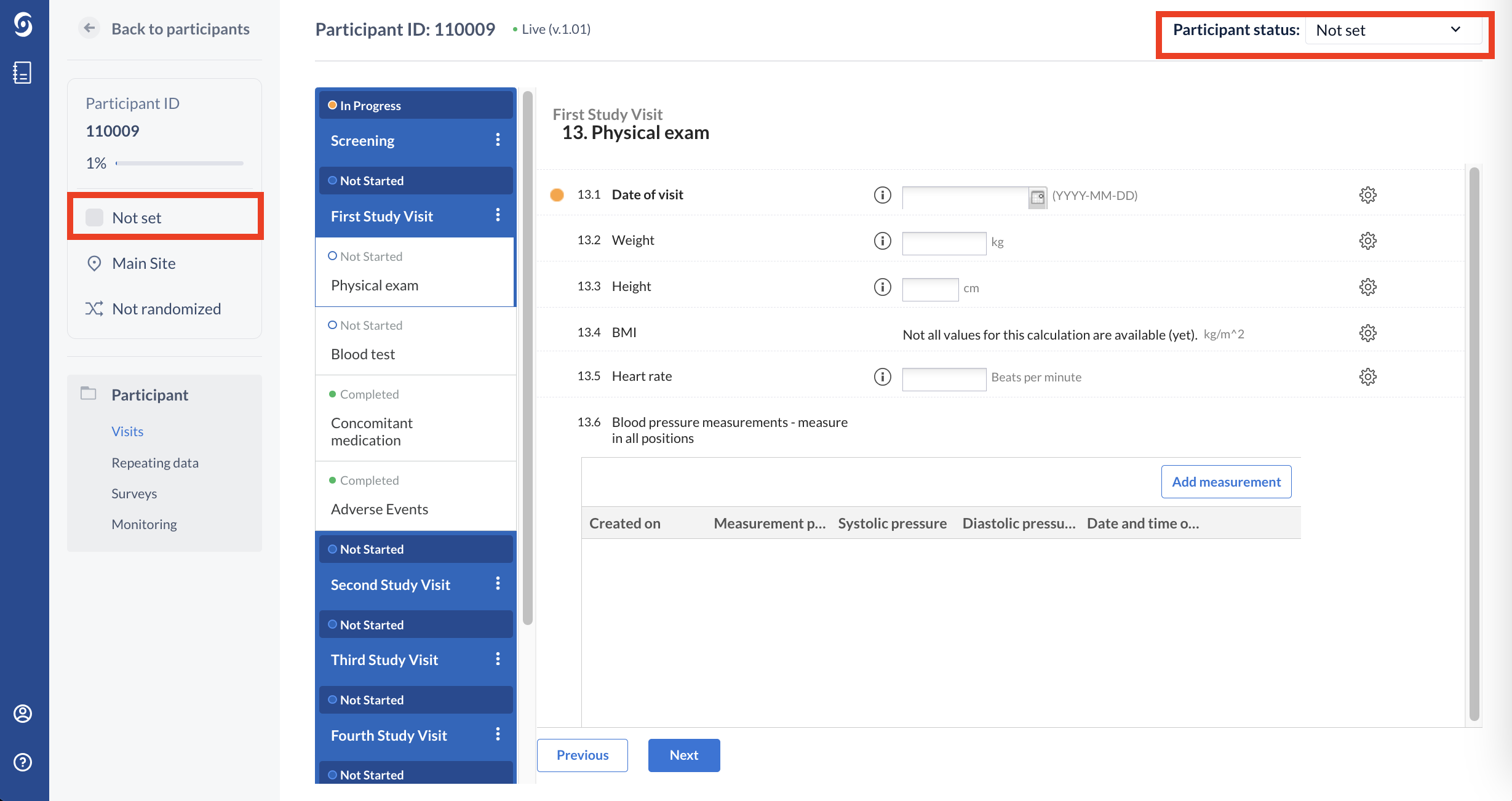
The Participant Status in CDMS
Tracking participants throughout the study lifecycle is critical. Whether processing individual participants, or summarized views of study progress, most users rely on this information for critical user-flows.
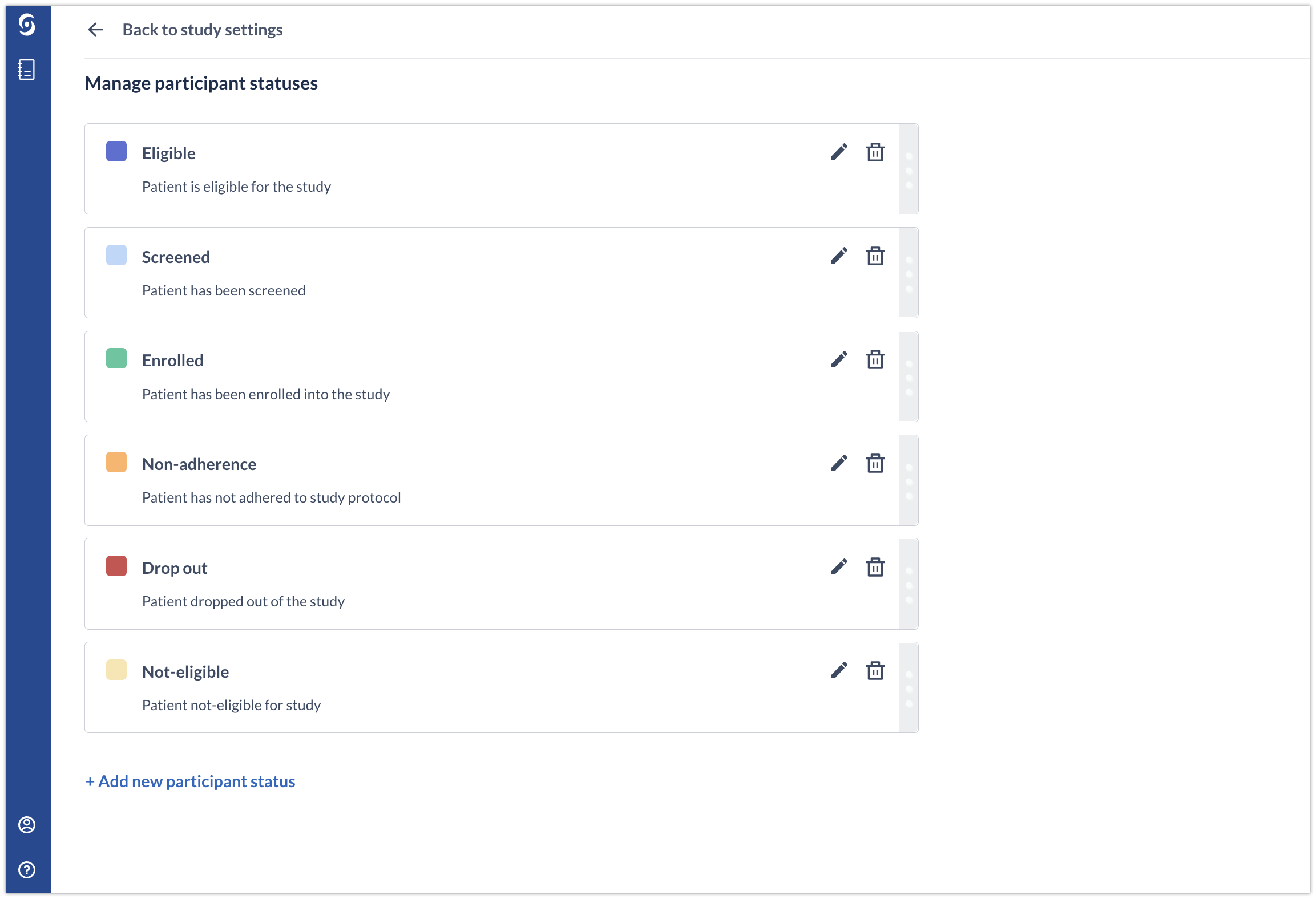
The participant status feature allows users to easily define and manage participants statuses. These can then be assigned to single or multiple participants and can be viewed in the participants overview.
The article below explains how to create and manage participant status for your study.
Creating a participant status
The study admins with the ‘Settings’ rights can create, update and delete participant statuses from Study Settings.
Follow the steps below to create a participant status:
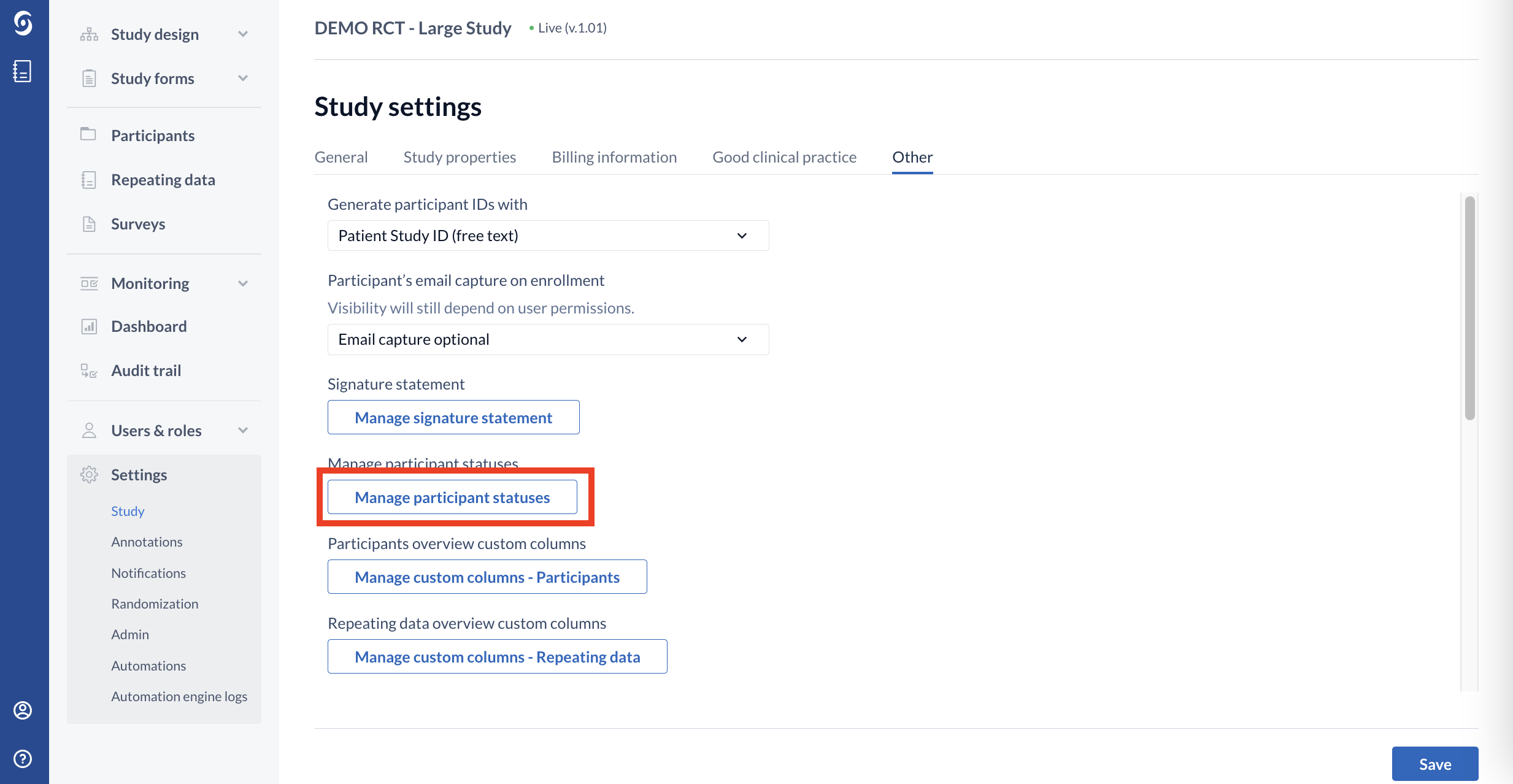
1. Navigate to the Settings tab and click the 'Other' section, then click the ‘Manage participant statuses’ button:
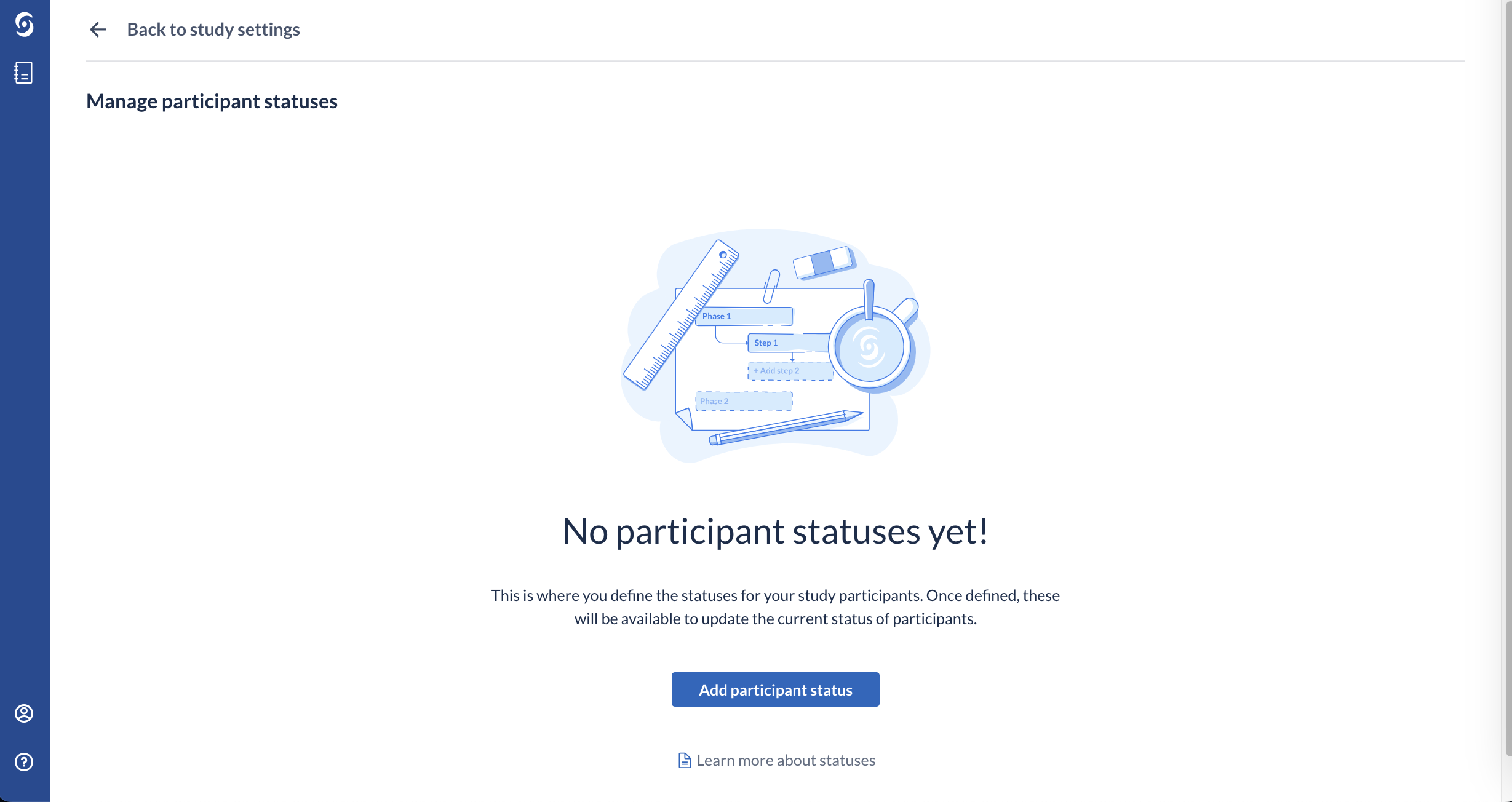
2. If it is the first status for your study, click on 'Add participant status' button:
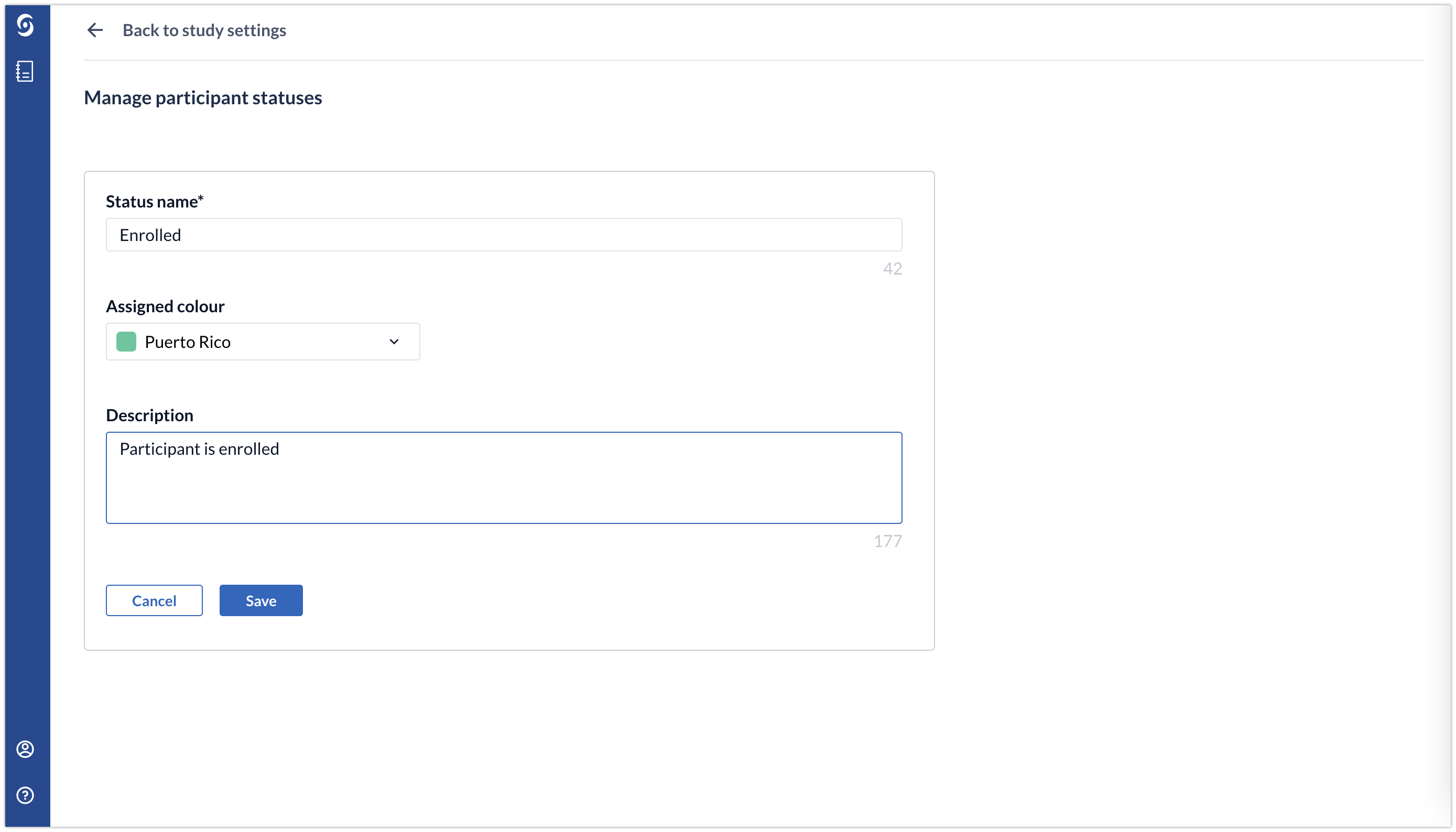
2. Add the properties for the status: status name, choose assigned and add description. Click 'Save' to save the status.
3. Add more status if required by clicking on the '+Add new participant status'.

4.Once a status is added, data entry users will be able to select the status in the participants view.
Editing or deleting an existing participant status
To edit an existing status, click on the pencil icon. To delete the status, click on the bin icon.
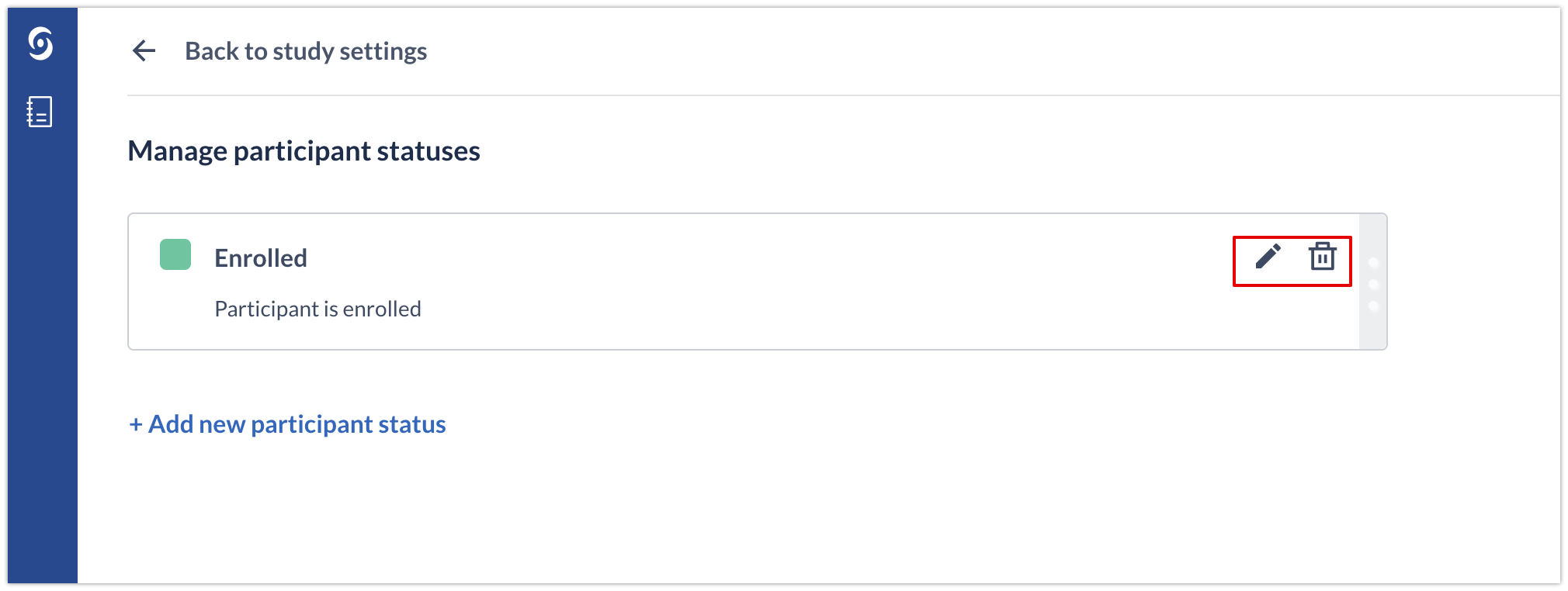
After removing a status, all participants that currently have this participant status will automatically get it reassigned to "Not Set".
You can drag and drop statuses cards on the “Manage participant statuses” page and the position/order of the statuses is saved automatically. The order of the status in Study settings matches the order of the statuses in the dropdown lists.
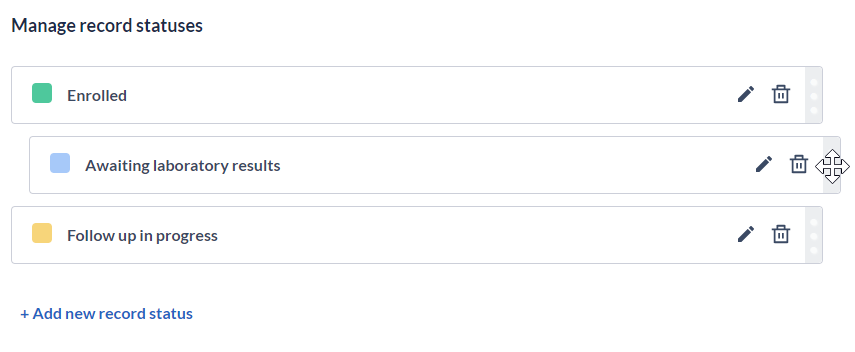
Once the participant status is defined in the Settings, study team members will be able to add it to participants manually. You can also update participant status when importing data as well as see participants statuses on printout and export files. To learn more about that, check the article Adding participant status.
Retrieving participant status via API
When a participant contains a participant status in Castor, this information will be included within the embedded code presented within the API as shown in the response below, for example when the endpoint to retrieve participant id:
{
"id": "120001",
"participant_id": "120001",
"ccr_patient_id": "",
"last_opened_step": "E31F92FA-11ED-4894-9396-5348B5ECBF0F",
"progress": 0,
"locked": false,
"status": "open",
"archived": false,
"archived_reason": null,
"created_by": "E31F92FA-11ED-4894-9396-5348B5ECBF0F",
"created_on": {
"date": "2022-02-22 13:58:44.000000",
"timezone_type": 3,
"timezone": "Europe/Amsterdam"
},
"updated_by": "E31F92FA-11ED-4894-9396-5348B5ECBF0F",
"updated_on": {
"date": "2022-11-22 15:03:00.000000",
"timezone_type": 3,
"timezone": "Europe/Amsterdam"
},
"randomized_id": null,
"randomization_group": null,
"randomization_group_name": null,
"randomized_on": null,
"_embedded": {
"participant_status": { // participant status is displayed within _embedded
"id": "enrolled",
"name": "Enrolled",
"_links": []
},
"site": {
"id": "E31F92FA-11ED-4894-9396-5348B5ECBF0F",
"site_id": "E31F92FA-11ED-4894-9396-5348B5ECBF0F",
"name": "Amsterdam Hospital",
"abbreviation": "AMS",
"code": "AMS",
"order": 12,
"deleted": false,
"country_id": 169,
"date_format": "Y-m-d",
"_links": {
"self": {
"href": "https://data.castoredc.com/api/study/E31F92FA-11ED-4894-9396-5348B5ECBF0F/site"
}
}
}
},
"_links": {
"self": {
"href": "https://data.castoredc.com/api/study/E31F92FA-11ED-4894-9396-5348B5ECBF0F/participant/120001"
}
}
}
If the participant does not contain a participant status, the extract will produce a null value and the embedded code will not be presented in the example response below:
{
"id": "110009",
"participant_id": "110009",
"participant_status": null, // participant status is set to null
"ccr_patient_id": "",
"last_opened_step": "1486CC24-C625-4BC4-86F5-0F40411D86CF",
"progress": 3,
"locked": false,
"status": "open",
"archived": false,
"archived_reason": null,
"created_by": "1486CC24-C625-4BC4-86F5-0F40411D86CF",
"created_on": {
"date": "2022-04-29 09:39:44.000000",
"timezone_type": 3,
"timezone": "Europe/Amsterdam"
},
"updated_by": "1486CC24-C625-4BC4-86F5-0F40411D86CF",
"updated_on": {
"date": "2022-11-02 11:58:00.000000",
"timezone_type": 3,
"timezone": "Europe/Amsterdam"
},
"randomized_id": null,
"randomization_group": null,
"randomization_group_name": null,
"randomized_on": null,
"_embedded": {
"site": {
"id": "1486CC24-C625-4BC4-86F5-0F40411D86CF",
"site_id": "1486CC24-C625-4BC4-86F5-0F40411D86CF",
"name": "Main Site",
"abbreviation": "CSH",
"code": null,
"order": 11,
"deleted": false,
"country_id": 169,
"date_format": "Y-m-d",
"_links": {
"self": {
"href": "https://data.castoredc.com/api/study/1486CC24-C625-4BC4-86F5-0F40411D86CF/site"
}
}
}
},
"_links": {
"self": {
"href": "https://data.castoredc.com/api/study/1486CC24-C625-4BC4-86F5-0F40411D86CF/participant/110009"
}
}
}