Manage study monitoring in SMS
Monitors and monitor visits can be managed at study level.
Monitor tab
You can manage the monitors and monitors visit on the monitor tab within the study.
Please note that normally the monitor tab is not available for a study. In order to enable it you need to go to "Review process" tab and set "Yes" in the "Is there monitoring?" field.
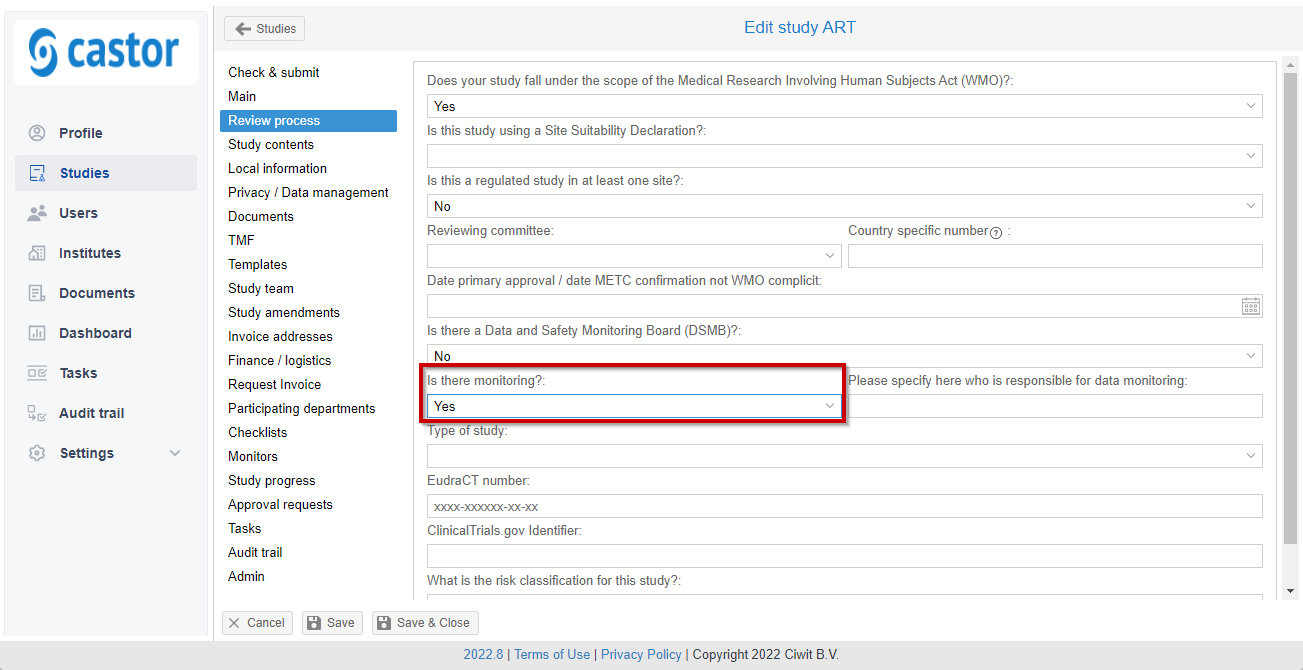
After that, refresh the page and you should be able to see the "Monitors" tab on the left side navigation panel of the study.
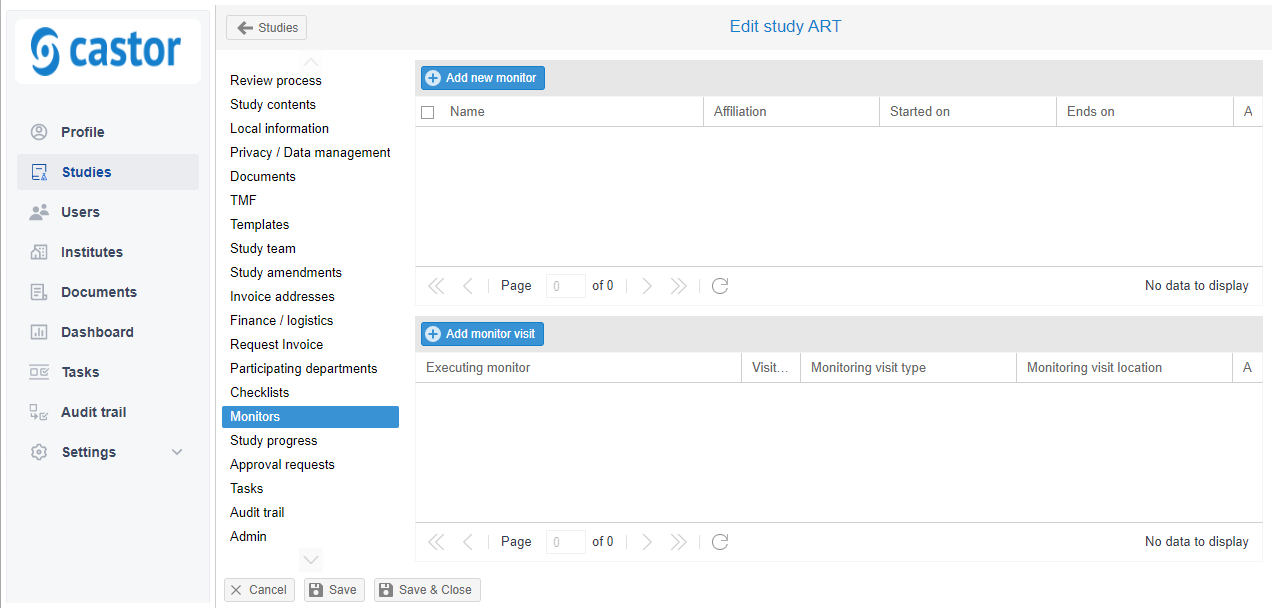
Add a monitor
- On the 'Monitors' tab.
- Click on 'Add new monitor'.
- Fill in the form.
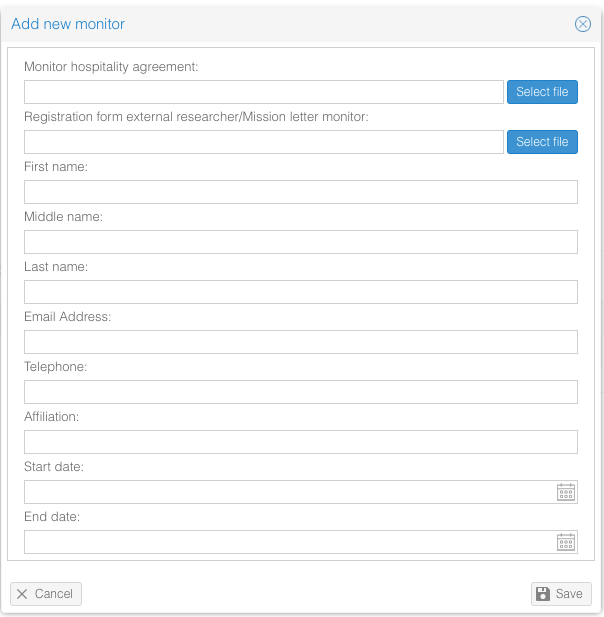
- Click on 'Save'.
The monitor is now added to the study. When adding monitor visits, the available monitors can be selected and associated to each visit.
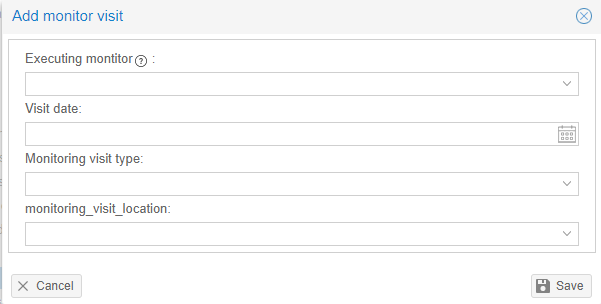
Add a monitor visit
- Navigate to the 'Monitors' tab.
- Click on 'Add new monitor'.
- Fill in the form.
- Please make sure that there is at least one monitor added to the study.
- Click on 'Save' to create the monitor visit.