Generate roles automatically (for departments and approvals) in SMS
Please note - user roles and user rights can only be managed by system administrators.
Castor SMS can generate roles automatically for approval requests and participating departments. A default set of user rights are automatically set up for these roles.
Create a new participating department
- Go to the 'Settings' tab.
- Go to the 'Roles and rights' tab.
- Go to the 'Participating departments' sub-tab.
- Click 'Add'.
Enter the department name.
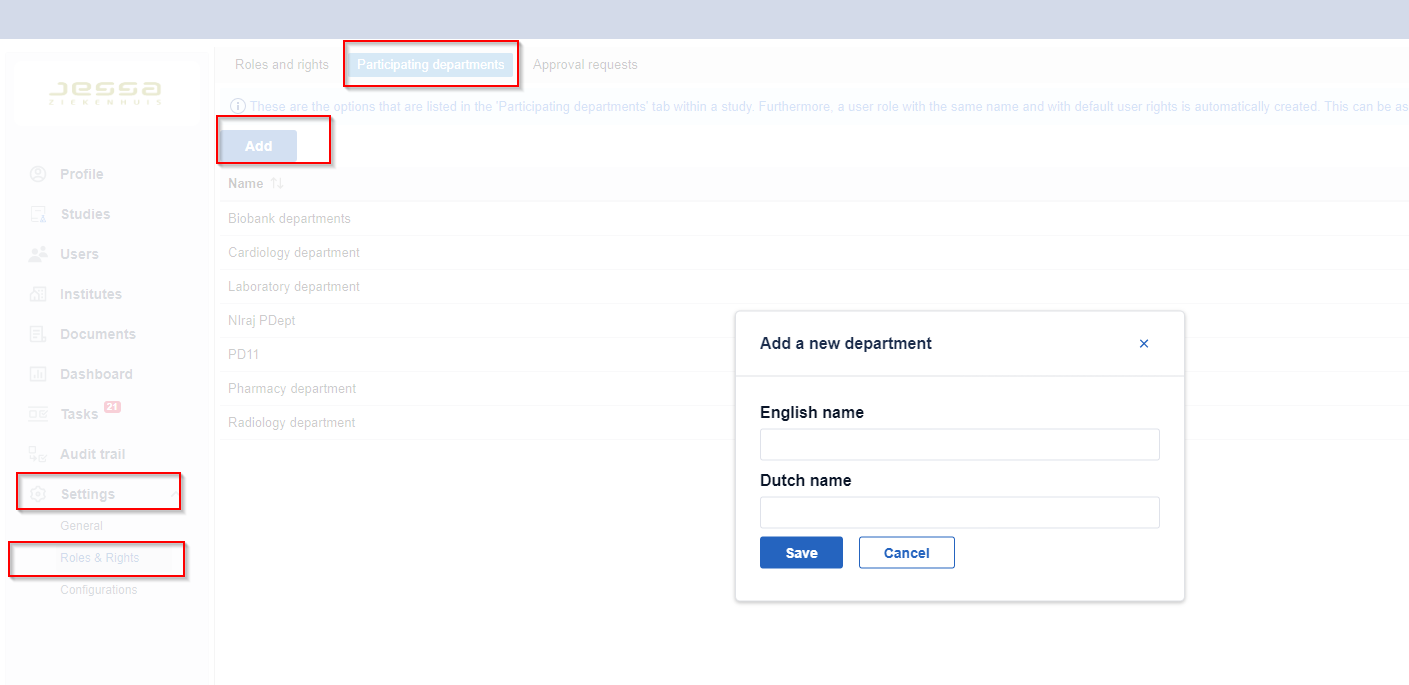
- Click 'Save' to add the participating department.
The participating department is added. This means that:
- This adds a new option to the ‘Participating departments’ tab within a study.
- This adds a new role in the 'Users' tab, this role is automatically provided with the following rights:
- 'View study details’ of studies that this department participates in.
- 'Documents’ tab - user can add, download and archive files.
- 'Participating departments’ tab - user can edit the data of their own department.
- 'Invoice addresses’ tab - user can add and edit invoice addresses.
Create a new approval request type
- Go to the 'Settings' tab.
- Go to the roles and rights tab.
- Go to the 'Approval requests' subtab.
- Click 'Add'.
- Enter the approval request name.
- You can link the approval request directly to a participating department from the ''Linked Participating Department'' dropdown menu.
- Choose if the users are allowed to create these approval requests.
- Choose if the users are allowed to edit these approval requests .
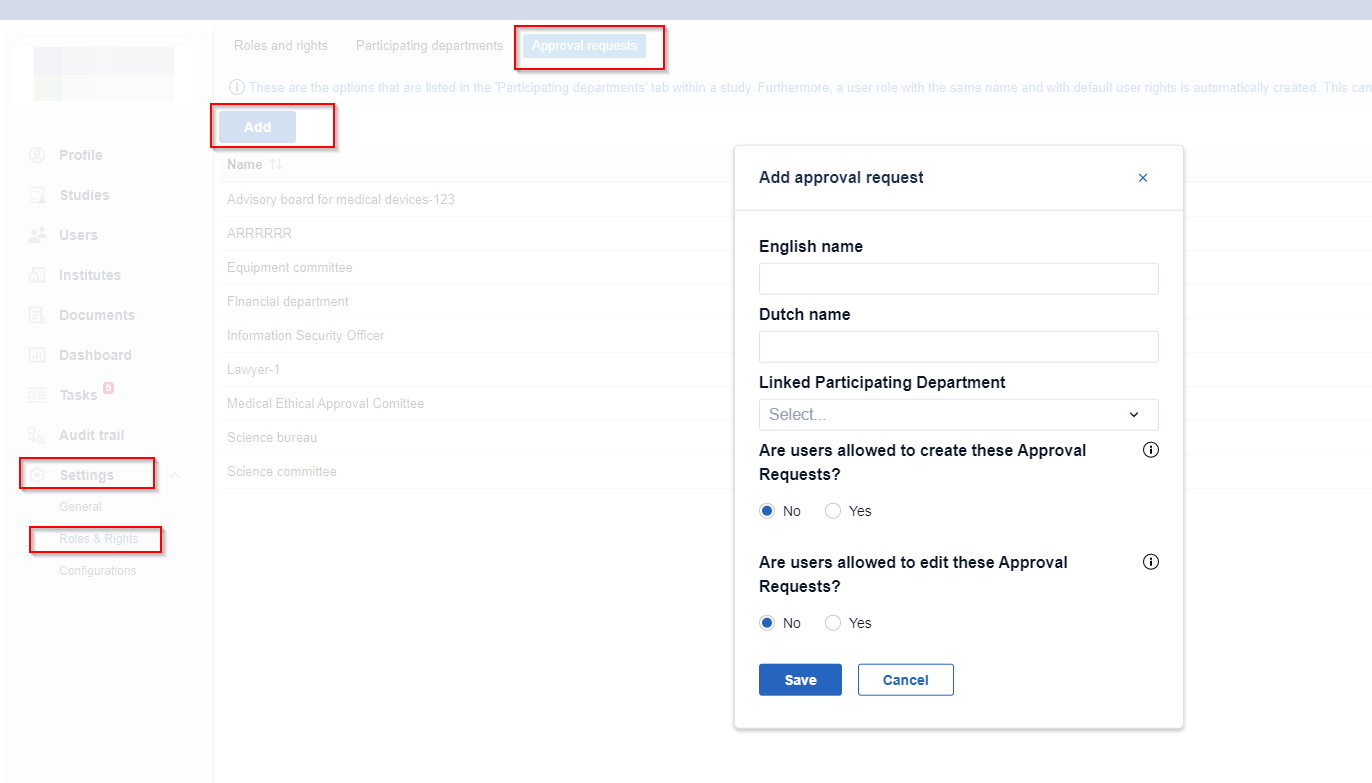
- Click 'Save' to add the approval request.
The approval request is added. This means that:
- This adds a new option to the ‘Approval request’ tab within a study.
- This adds a new role in the 'Users' tab, this role is automatically provided with the following rights:
- 'View study details’ of studies that this department participates in.
- 'Documents’ tab - user can add, download and archive files.
- 'Approval requests' tab - user can edit the details of their own approval request, and can add new approval requests;
- 'Dashboard' menu - user can access the approval request view of the dashboard.
It is not possible to remove a Participating department option or Approval request option if:
- The option is being used on a study.
- The role is being used by a user.