Export signature and participant information from eConsent
Users with the ‘Study Investigator’ or ‘Study Monitor’ role are able to generate an export of signature information.
To export the signature information, follow the steps below:
1. In the ‘Participants’ overview, click on the ‘Export’ button:
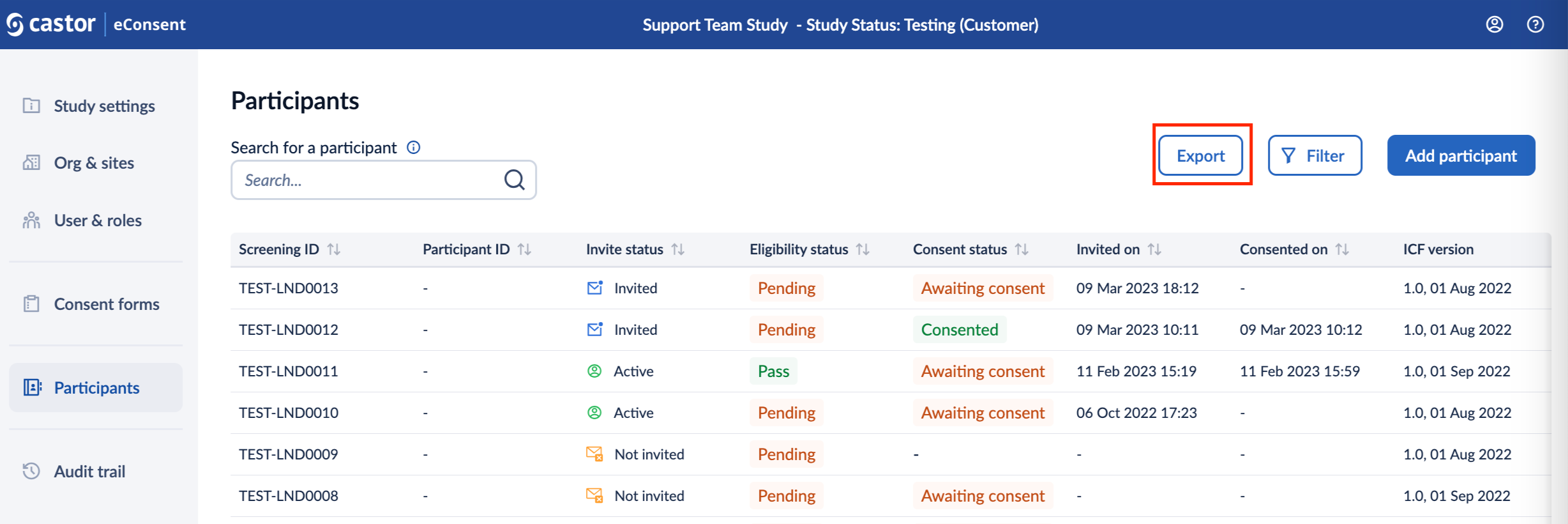
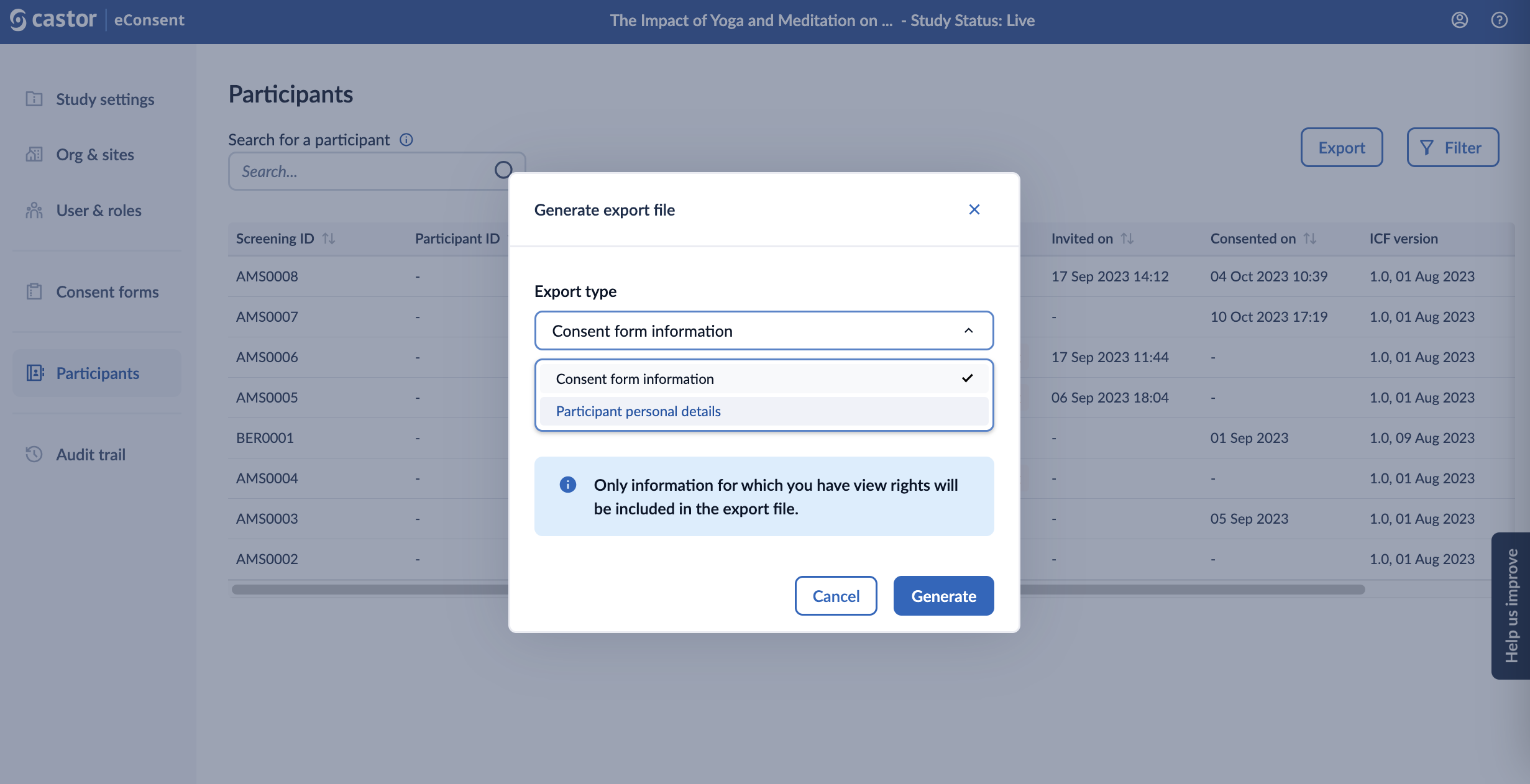
2. In the ‘Generate export file’ pop-up window, in the ‘Export type’, select ‘Consent form information’ or ‘Participant personal details’ option. Click on the ‘Generate’ button to proceed with the export. Only signature information for which you have view rights will be included in the export file.
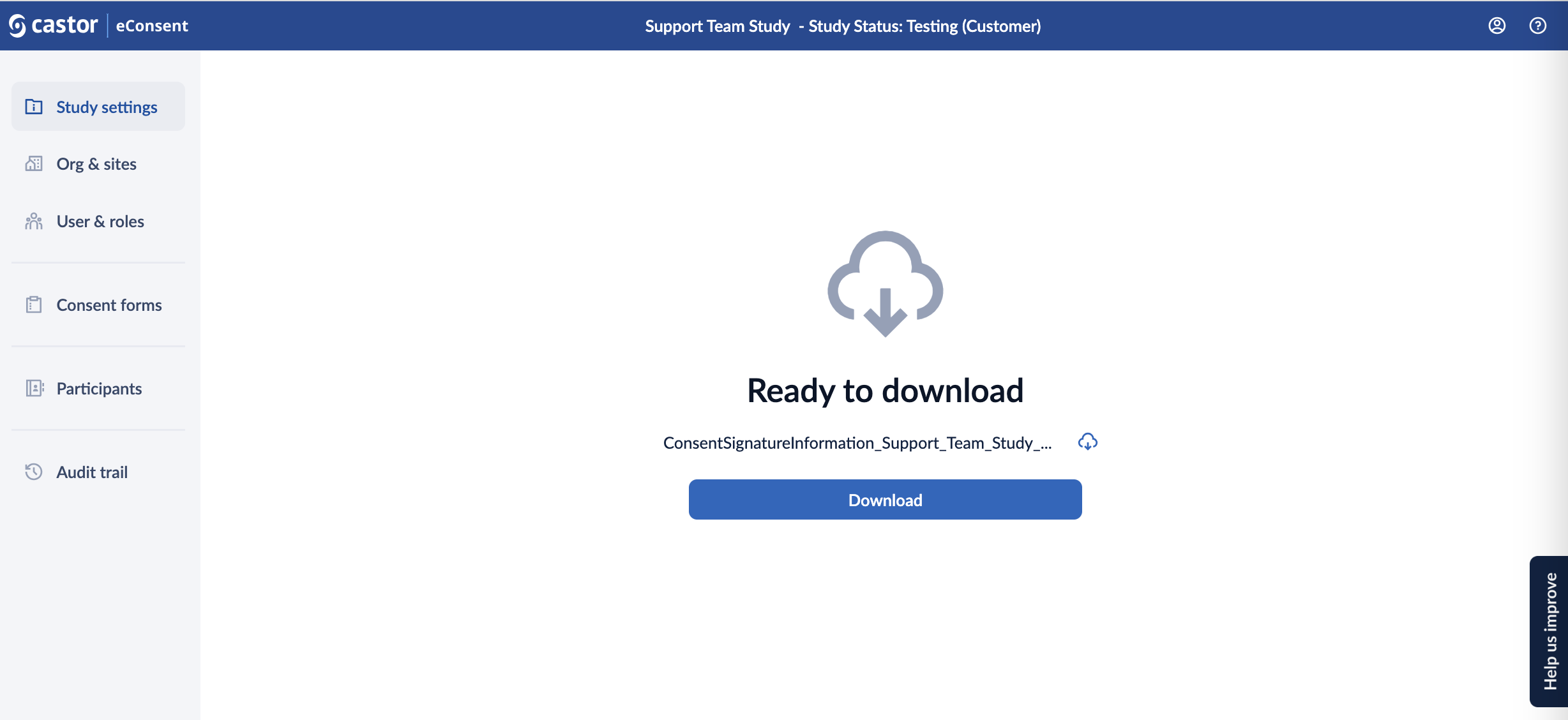
3. Once the export is generated, the requester is notified by an email that includes a personal download link.

The generated export can then be downloaded from within eConsent by clicking on the ‘Download’ button or pressing the ‘Download’ button within the email in which case you will be redirected to the eConsent platform to download the file.
3. The consent form export file will contain the signature information:
- Screening ID
- participant ID
- participant created on
- participant created by
- Invite status
- Site
- Site country
- Unique ICF ID
- Language
- Sent on date
- Consent form
- Version number
- Version date
- IRB approval date
- ICF consent method
- Consent date
- ICF filename
- Consent status
- participant name
- participant email
- participant signature status
- participant signature date/time
- PI name
- PI email
- PI signature status
- Information on any additional signatures and signature labels as defined in an ICF
The file contains only the optional signature agreements (checkbox fields).
4. The participants personal details export file will contain the following details:
- Screening ID
- Participant ID
- Consent status
- Site
- First name
- Last name
- Email address
- Date of birth
- Gender
- Phone number
- Street
- Addition
- City
- State/province/region
- Zip code
- Country