Managing overseeing organization and sites in eConsent
Table of Contents
The 'Overseeing Organization & Sites' overview allows to change the details of the main organization, add new study sites and manage existing ones.
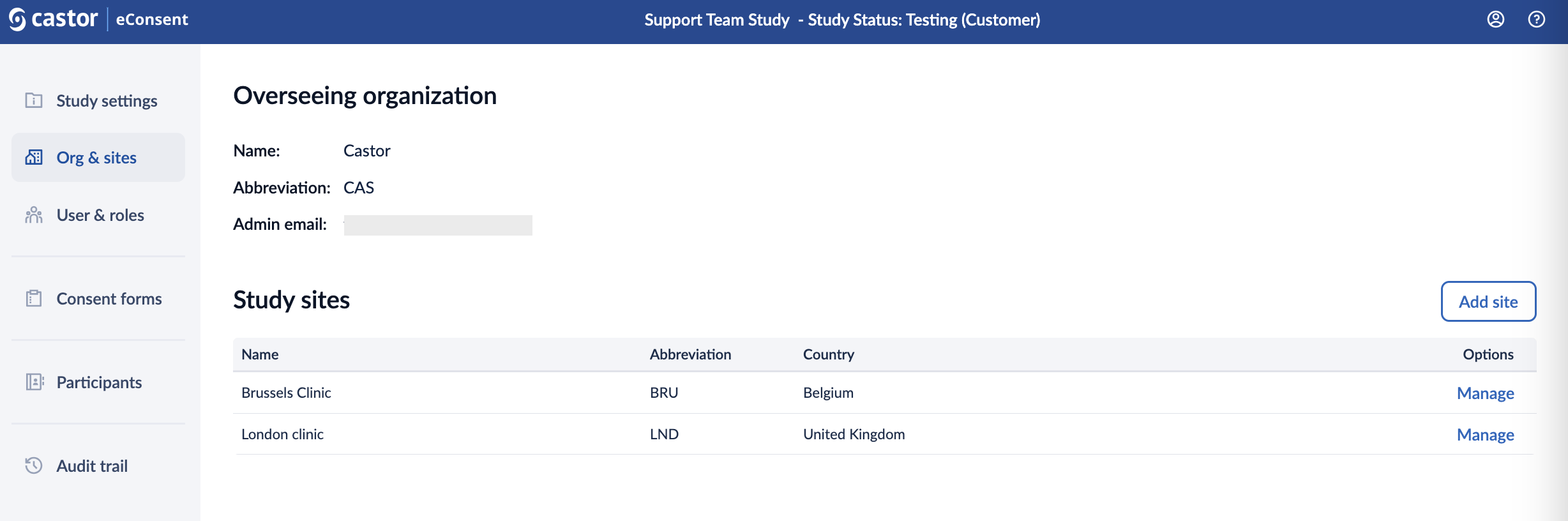
Overseeing Organization
In this section, you can view the following details related to the overseeing organization: Name, Abbreviation, Country, Admin email.
Study sites
In the study sites section users with the ‘Study Admin’ rights can manage existing sites and add new sites in the study.
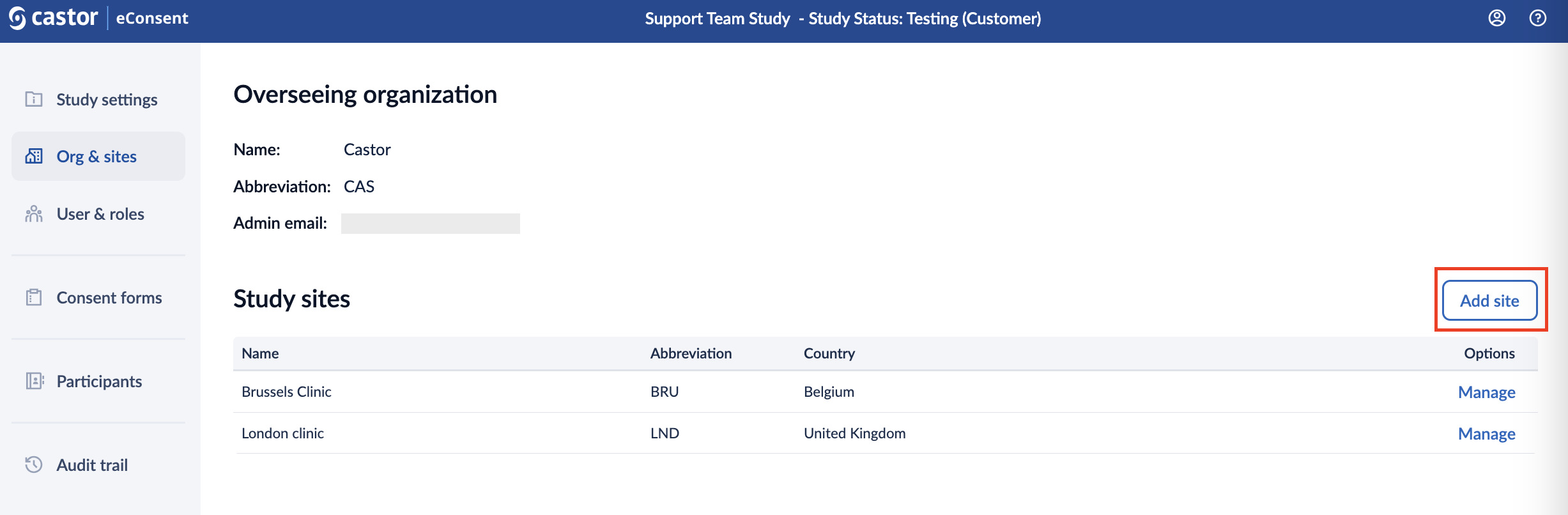
To add a study site:
1. Click the ‘Add site’ button:
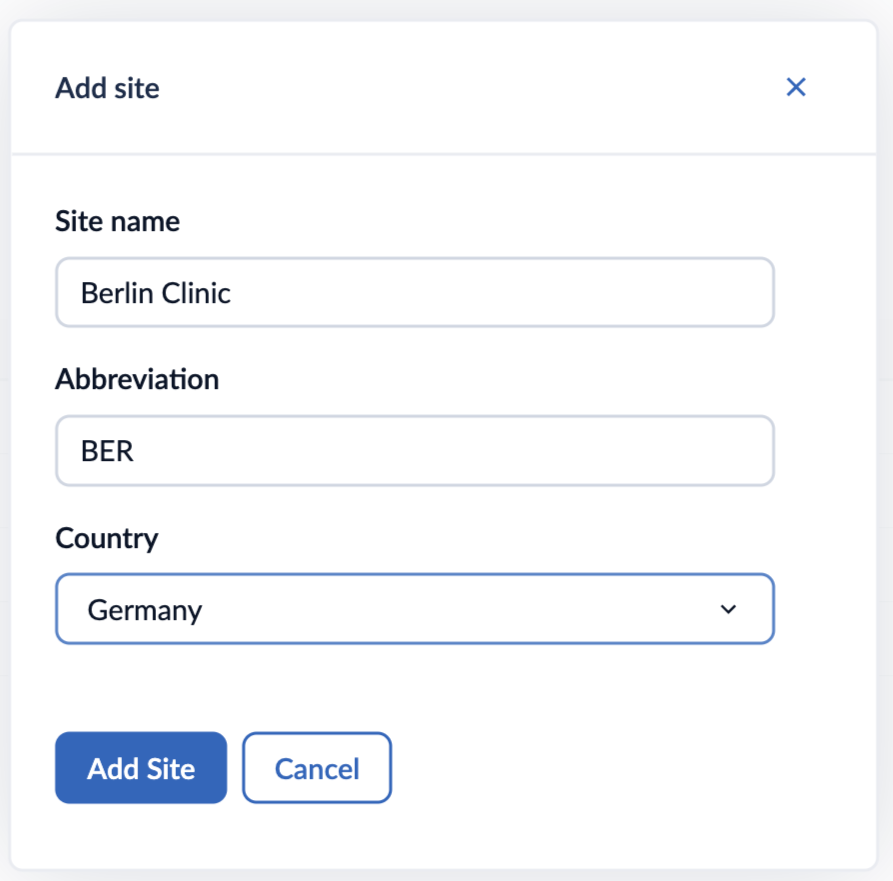
2. Enter the site details such as Site name, Abbreviation, and Country
3. Click ‘Add site’:
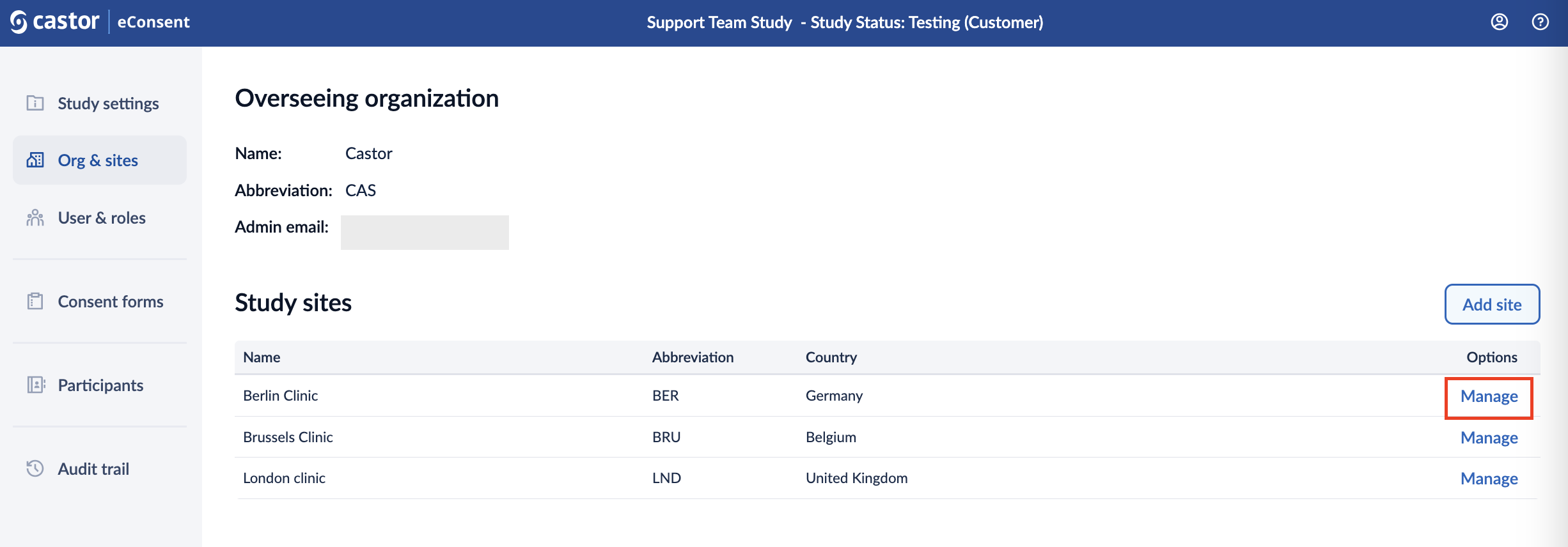
To edit the site details or to delete a site:
1. Click on the ‘Manage’ icon in the ‘Options’ column.
2. A pop-up window will appear where you can adjust the site details accordingly.
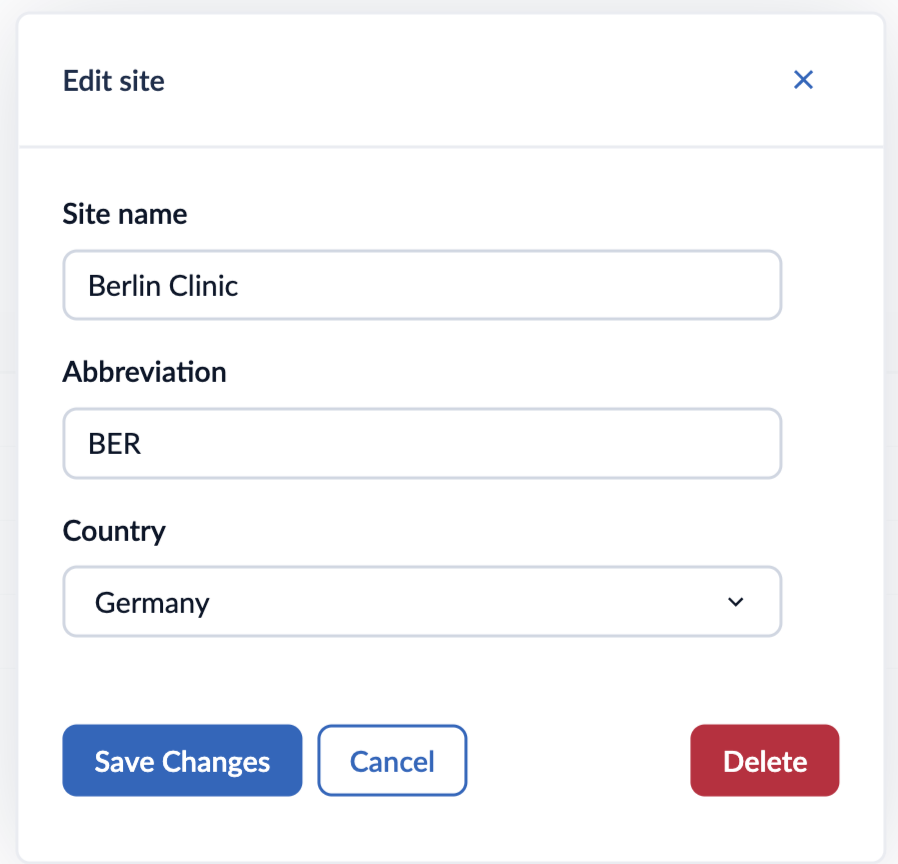
3. After editing the site information, click on ‘Save changes’ to apply the changes.
4. If you would like to delete the site, click on ‘Delete’ button. A confirmation message will appear.
It is not possible to delete a site, if it is in use by a participant, by an informed consent form or by a user.