Access your study in eConsent
Before you are able to access your eConsent study you will first need to be invited and create an account for eConsent. For more information on this subject please see “Create an account and log in into eConsent” article.
In order to access your eConsent study after registration has concluded you will need to access the following url’s bases on the location of your study, US or Europe.
- US eConsent - https://us.castorconsent.com/
- EU eConsent - https://eu.castorconsent.com/
Study data is only stored on one of these servers and only one server location can be accessed at a time. If you don’t see any studies listed please make sure to check our other server location.
You can toggle between the two study locations by selecting the Europe or USA icons at the top of the page.

Upon entering eConsent there will be two sections, Invitations and Studies. The Invitations section will only appear if you have been sent an invitation and have not yet accepted or rejected the invitation. Before accessing any studies you will first need to accept the invitation.
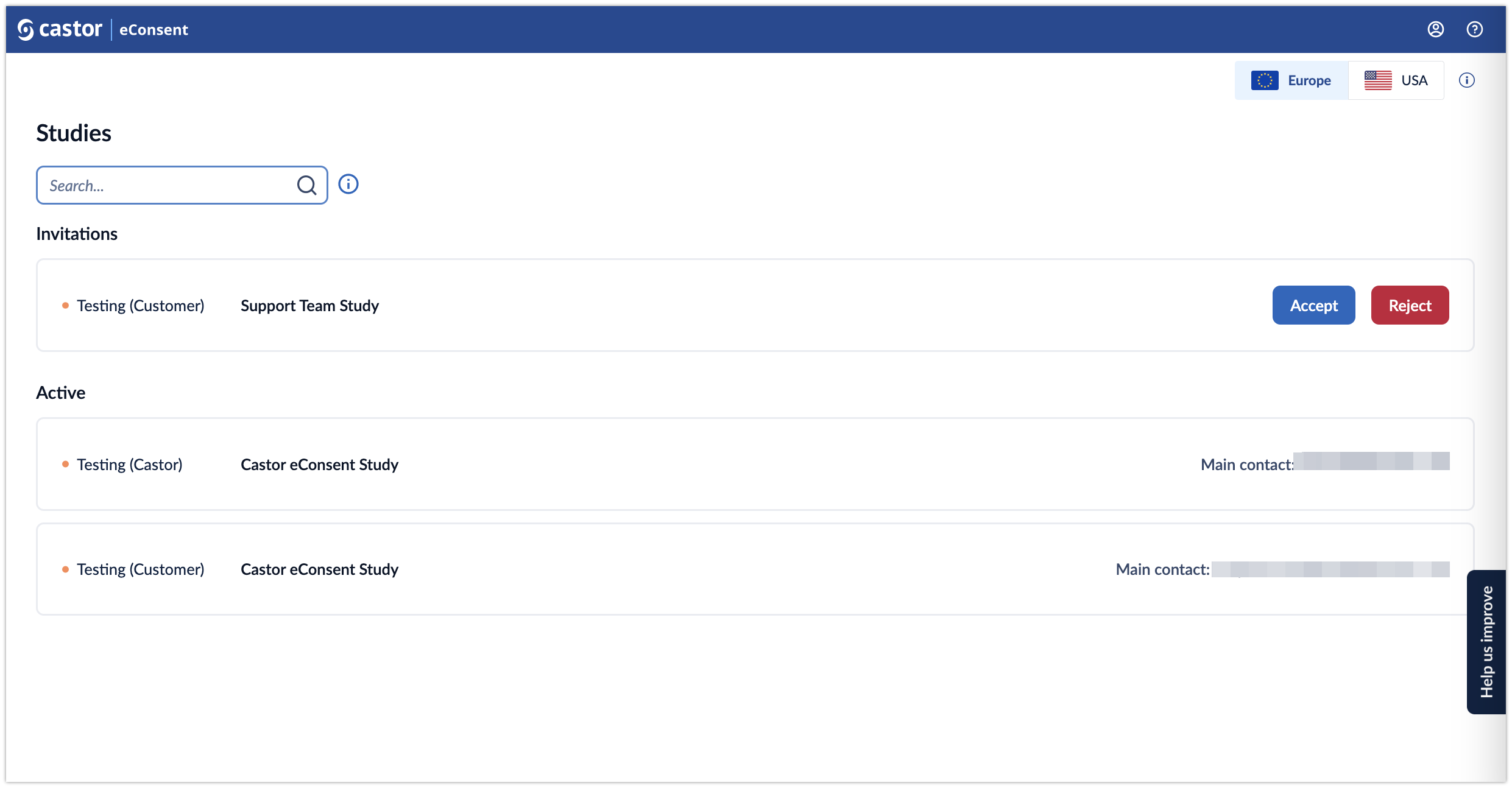
Once the invitation has been accepted your study will be listed under the heading of ‘Active’. To enter the study select the study name you wish to enter.
Studies overview consists of the following elements:
- Servers: Here the data storage locations are displayed. There are two server locations available: Europe and the United States. Study data is only stored on one of our servers and only one server location can be accessed at a time. If you don’t see any studies please make sure to check a different server location.
- Search bar: it is possible to search by organization name, study contact email address or study name
- Invitations: If you are invited to a study, but you have not accepted the invite yet, the study will be listed in the top panel. It is possible to either accept or reject an invitation to a study. Once you have accepted the invitation, you will be able to open a study.
-
Studies: once you have accepted an invitation to a study, it will appear in the ‘Active’ list. The list displays all studies you have access to. For each study, the following information is shown:
- Status: Testing (Castor), Testing (Customer), Live. Read more about what each status means in the article: Study status in eConsent
- Study title: name of the study
- Main contact: main contact of the study. If the main contact has been invited to the study, but have not accepted the invitation yet, the status will be ‘Main contact invited’
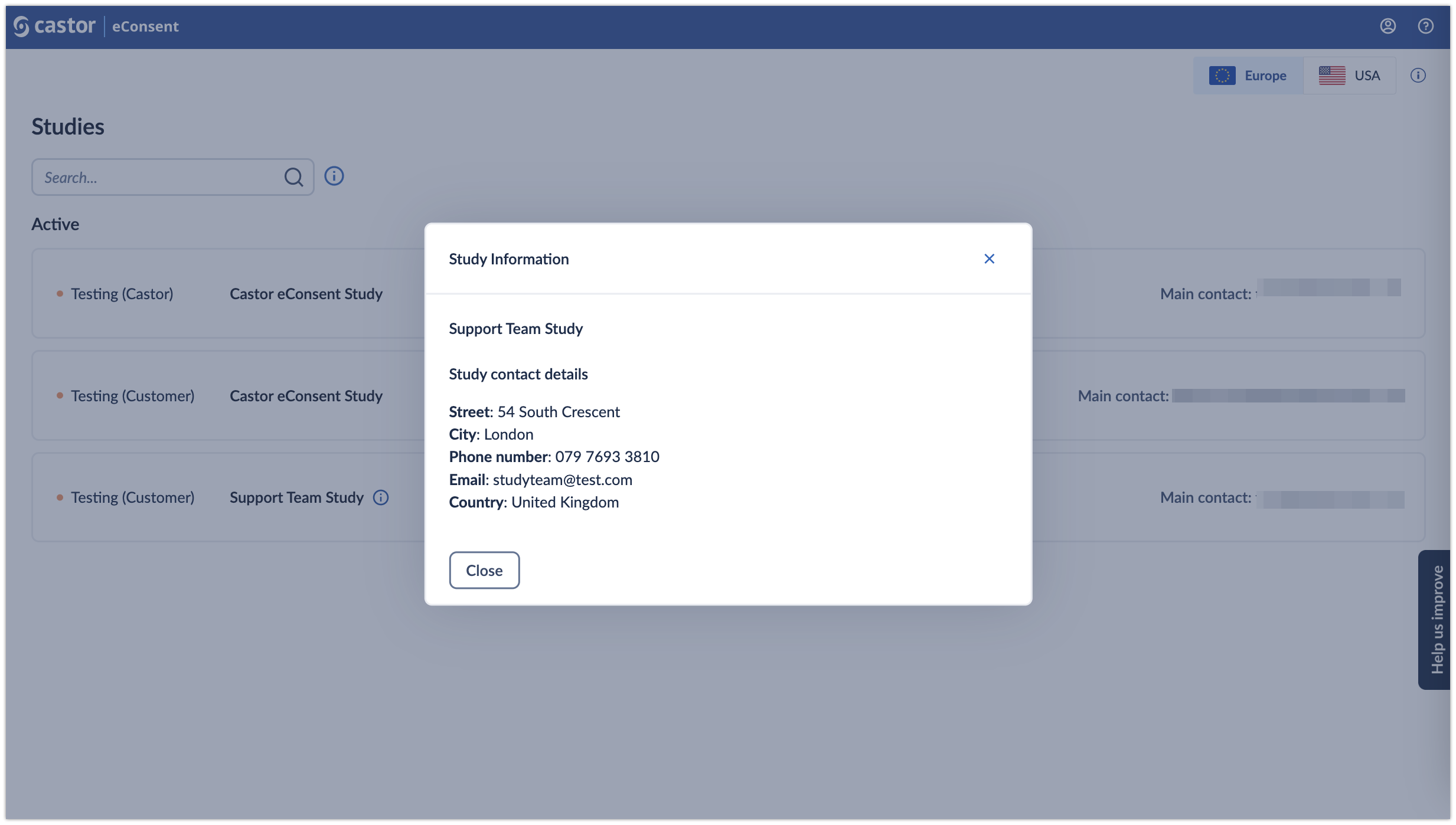
- Study information: by clicking on the (i) icon, it is possible to view additional study information