Study status in eConsent
The current status of a study is visible on the Studies page, as well as when opening an individual study. See eConsent: Studies Overview for more information.
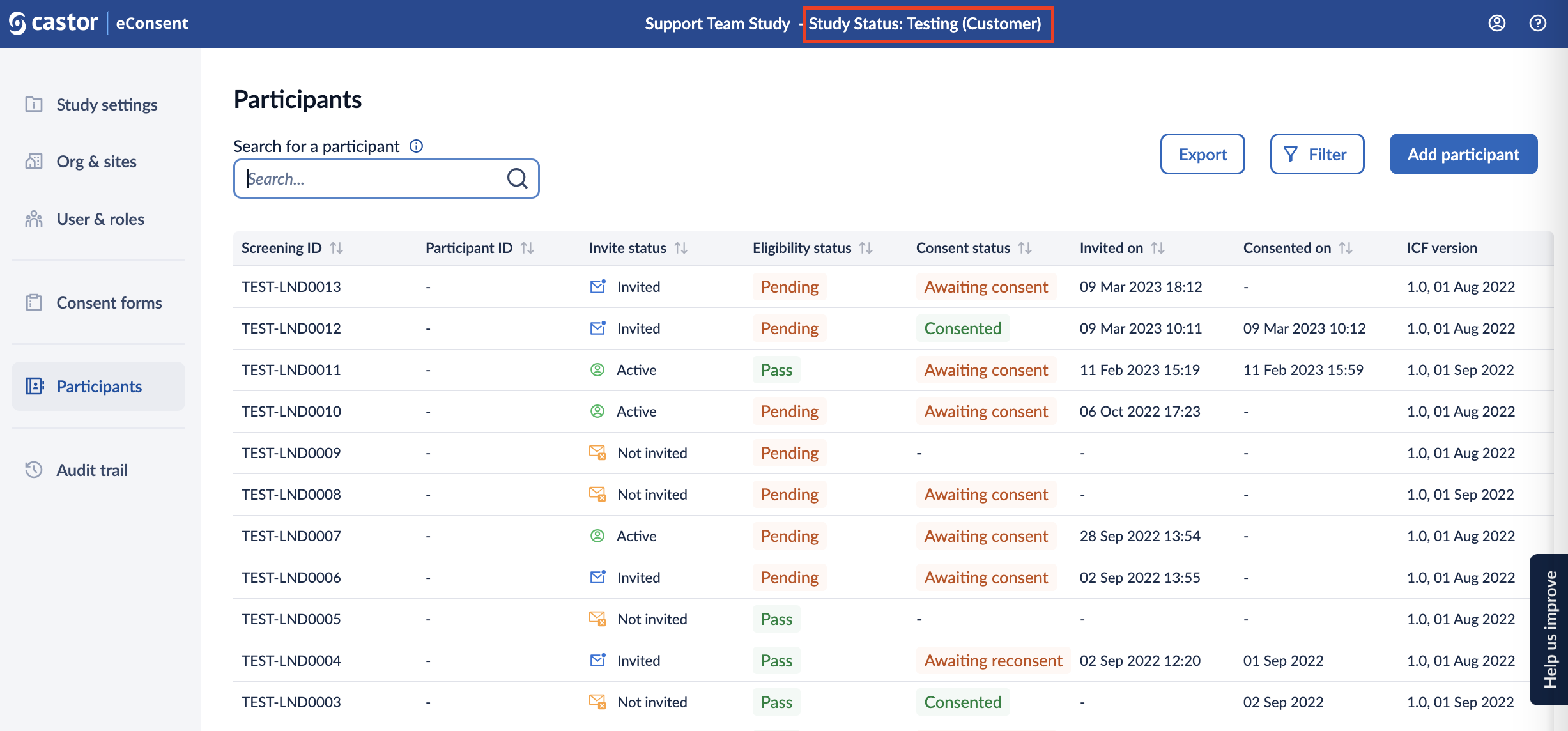
Each study progresses through the following statuses:
- Testing (Castor): The study is being tested by Castor. Audit trail events during this study status are marked with the status 'Testing (Castor)'.
- Testing (Customer): The study has passed the quality checks by Castor and is handed over to the customer to perform User Acceptance Testing (UAT). Audit trail events during this study status are marked with the status 'Testing (Customer)'.
- Live: The study has passed the quality checks by Castor and UAT by the customer. All the participant records that were created during both 'Testing' study statuses are automatically archived. Audit trail events during this study status are marked with the status 'Live'.
The Study status can only be changed by an organization admin if you are building the eConsent study yourself or a study admin.
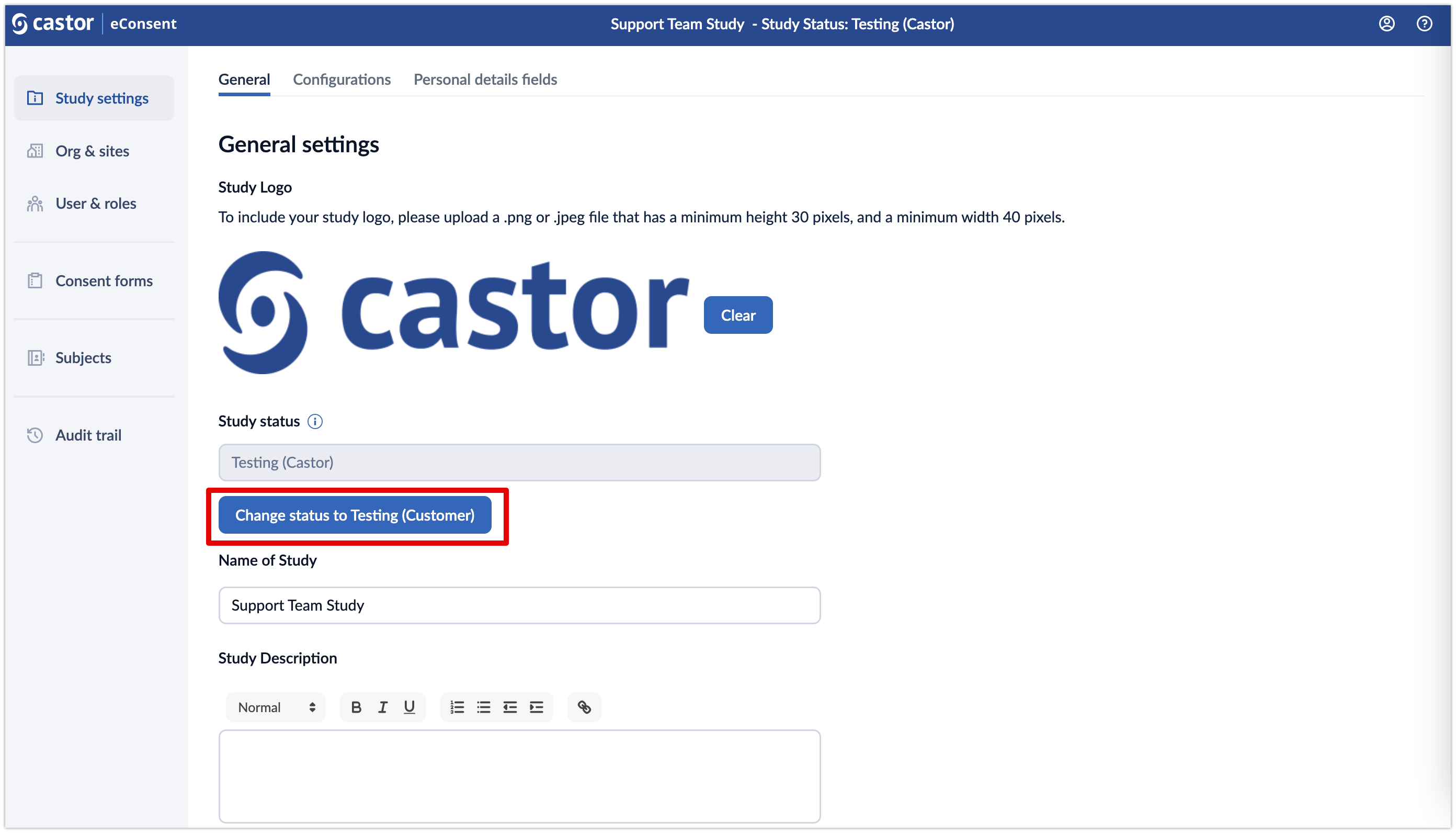
Once the study status has been changed, it is not possible to revert to the previous status.