Audit trail in EDC/CDMS
Learn the importance and process of maintaining an audit trail in Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS).
Table of Contents
The Audit Trail shows all changes that are made to your study. This includes changes both during building the form and during data entry. The Audit Trail will be visible if a study team member has all management rights
The Audit Trail
The audit trail enables you to review changes to study settings and study data. You can filter the Audit trail on various events such as, but not limited to: survey package is opened, a study is set to live or changing of user rights. Beside this you can see who performed the action and what participant was affected. For all filtering options please view the "Filter on event type" drop down in the Audit Trail tab.
To see all changes made in the eCRF, navigate to the 'Audit Trail' tab:
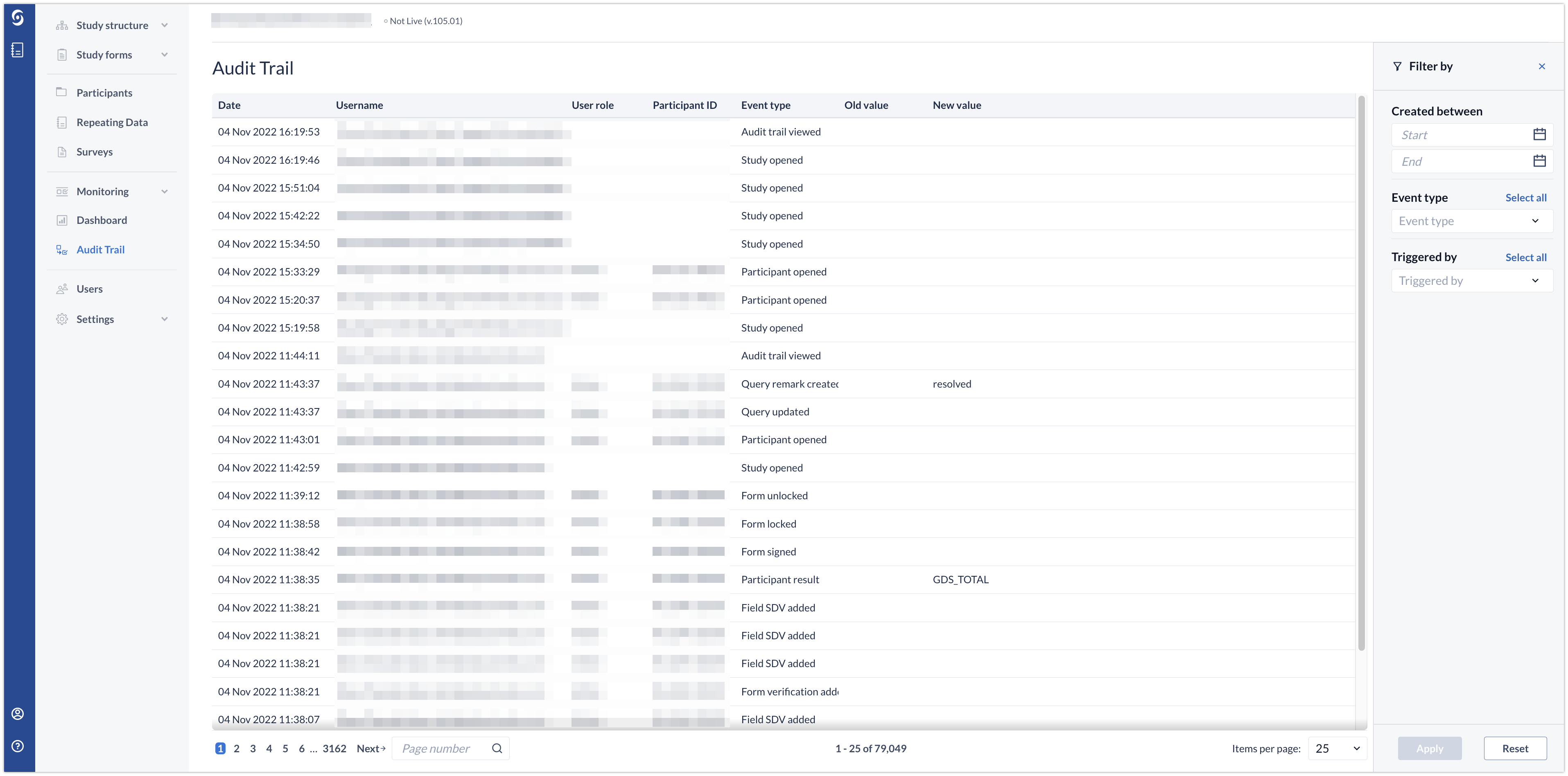
All the changes that are made to the study are displayed in the "Audit Trail" tab.
- Here you can filter the Audit Trail on the event type, user that triggered the event, or date.
- An overview of all events are given relating to the filter. If no filter is selected all events are shown.
You can also use the multi-select option in the “Event type” and the “Triggered by” drop-downs:
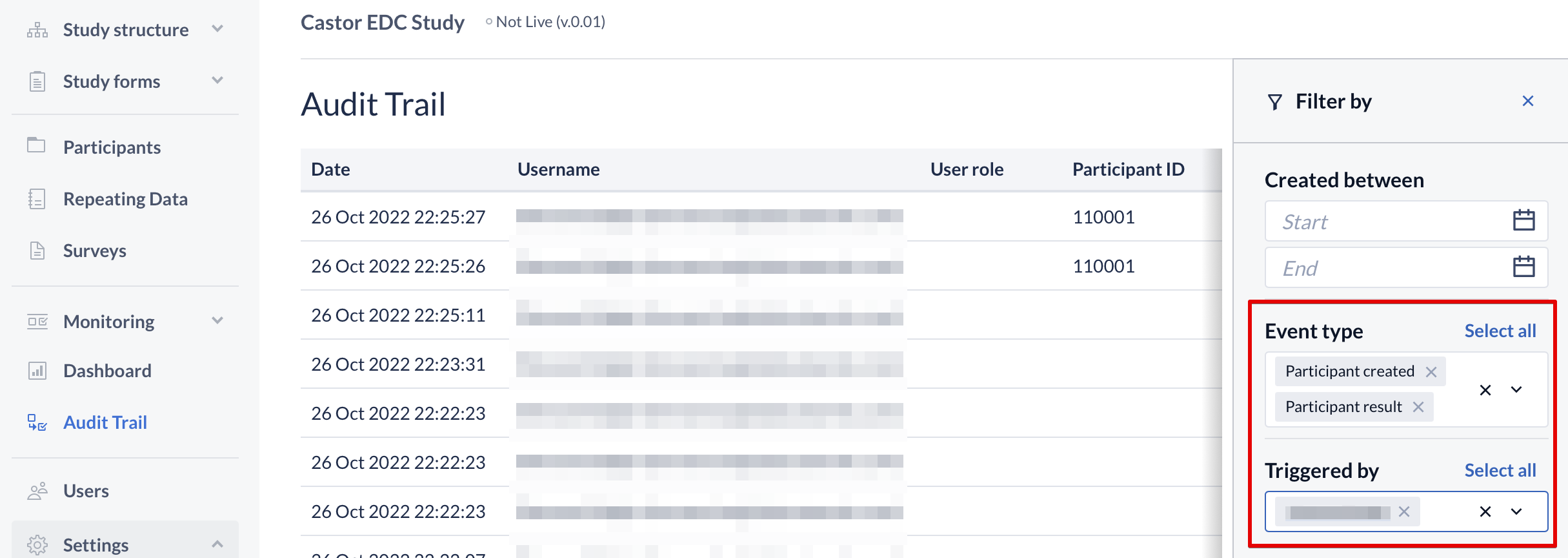
While reviewing the event monitoring, the user can see old and new values. This gives a better overview of the trial progress and each data point's history.
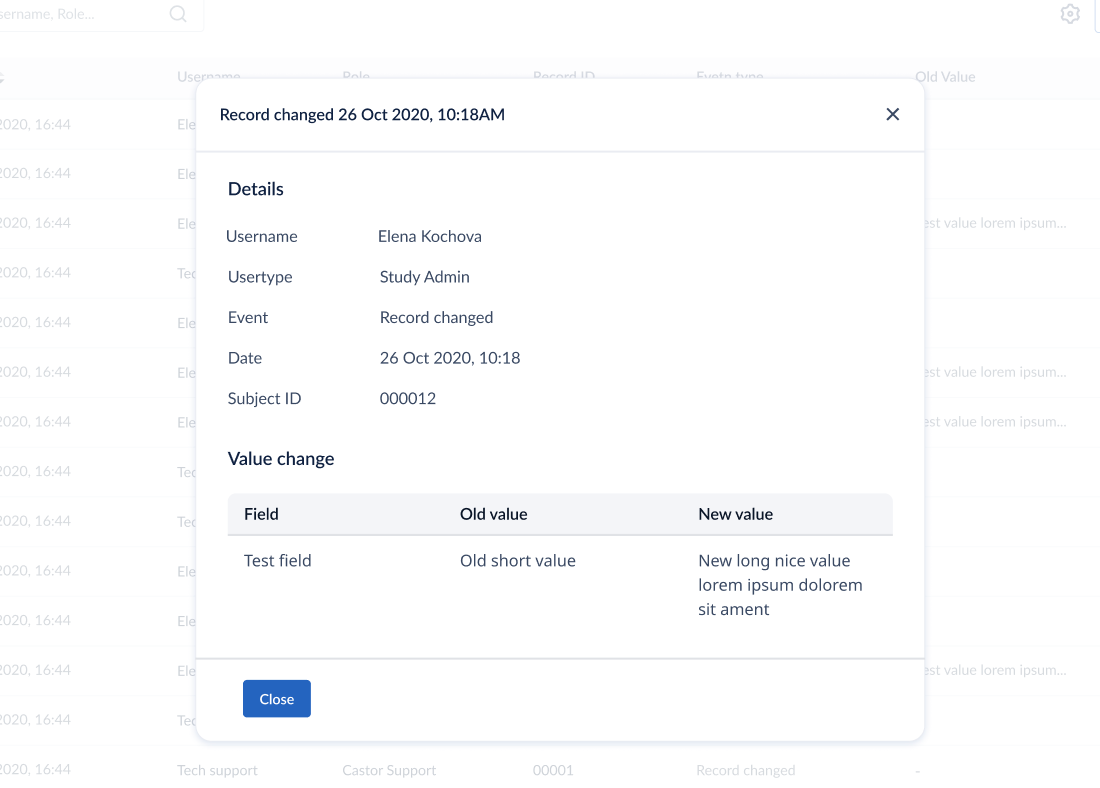
See changes made in a single field
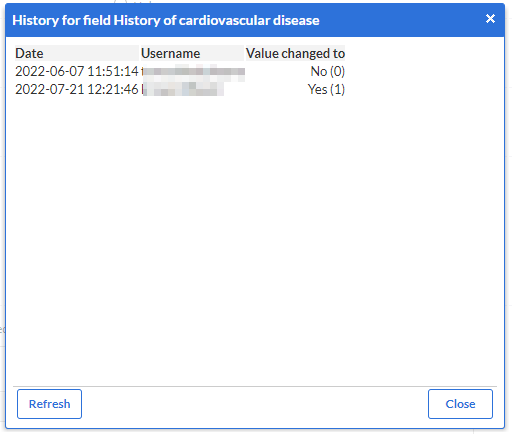
You can also view the audit trail for a single field from within the study view:
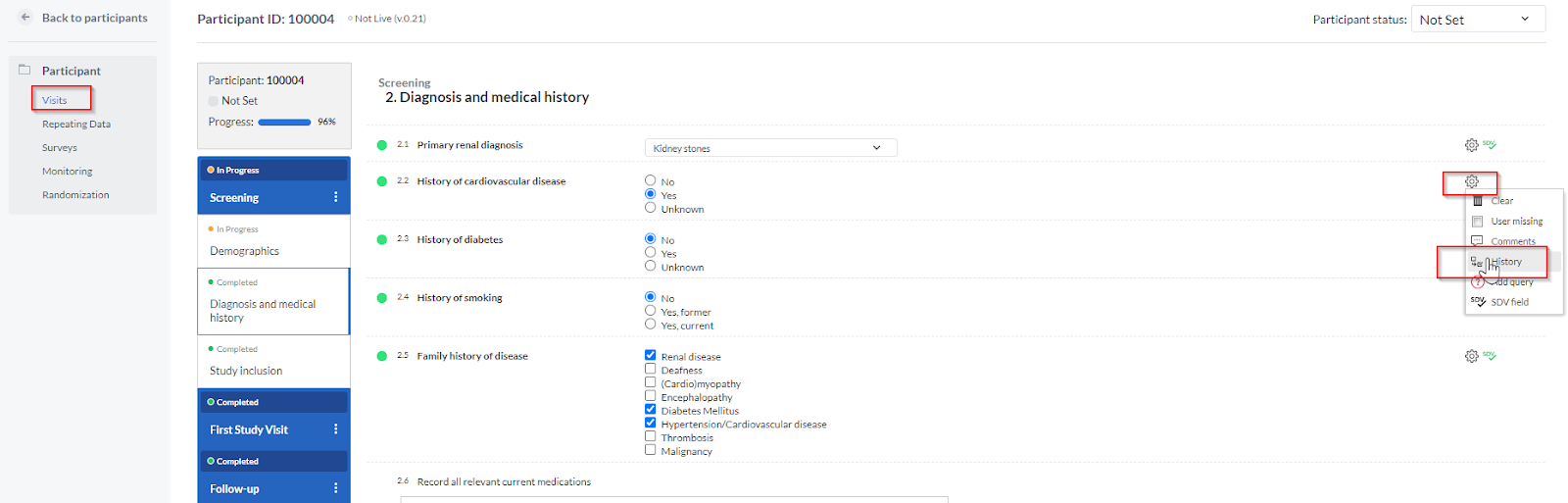
To see the changes that are made to a specific field click the cogwheel next to a field in the study overview. A popup will appear detailing the changes to the field. The date, the user who changed the field, and the new value are all displayed:
Please note that the test site and the site that is set up in the study creation form are not counted as "site created" event. Therefore, only the sites added after the creation of the study have this event.
Audit trail for Signatures
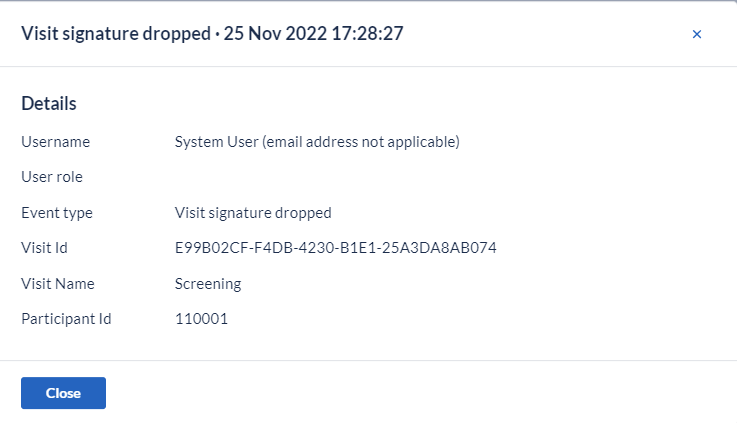
For the 'Participant signed' & 'Participants signature dropped' events, these are logged in the system both when the event was triggered manually, by a logged in study user, as well as when it was triggered automatically by the system, as a direct result of another related action such as structure changes.
Every time a digital signature is automatically applied or removed by the system due to another action or event that took place in the system, specific and independent audit trail event(s) is (are) logged in the Audit trail for the action(s) carried out by the system. These details are only stored on the events that were initiated by actual study users.
Every time a logged-in study user applies or removes a digital signature, specific and independent audit trail event(s) is (are) logged in the Audit trail for the action(s) performed by the user.
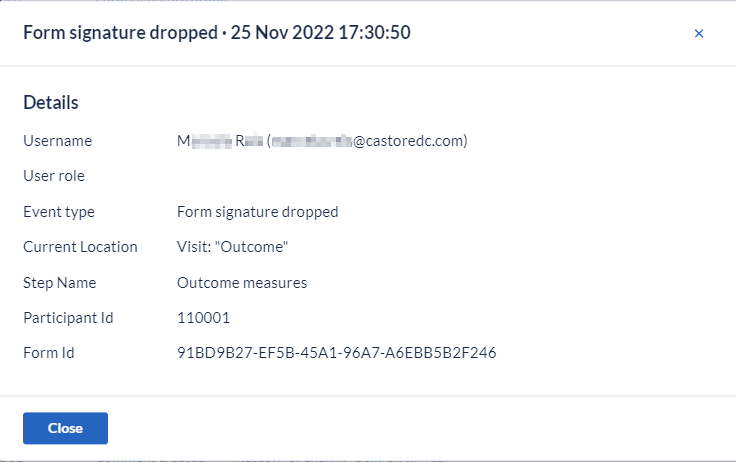
When you manually sign/unsign a participant the archived repeating data instances do not get affected. When you sign/unsign a participant using other scenarios e.g. structural changes, the archived repeating data instances are also affected and logged in Audit Trail.
When a repeating data instance is unarchived, the sign logic is recomputed and updated automatically and any relevant Audit Trail events are logged in the Audit Trail.
Retrieve Audit trail via API
It is possible to retrieve the Audit trail via API /study/{study_id}/audit-trail/ endpoint. The authorized users can retrieve the selected time range of the audit trail events or filter by event types and user IDs.
Due to changes in the audit trail architecture, Queries and Comments created before the 1st of August 2020 are not available via this endpoint. Nevertheless, these are always accessible via the Audit Trail user interface.
When accessing the Audit trail, the date and time are displayed in ISO8601 format (YYYY-MM-DD HH:mm: ss).
Exporting the Audit Trail
The audit trail export typically does not include the study build, i.e., the creation of visits, forms, fields, etc. We provide audit trail export for free once at the end of the study in the CSV format. Please note that for additional audit trail exports, there will be an additional fee.
Events that are included in the audit trail export are:
- participant created and archived
- data entry events (only study and repeating data)
- comments and queries
- repeating data created and archived
- verifications (and drops)
- signatures (and drops)
- locks and unlocks (visits/forms/participants)
- user permissions
- export created
To request an audit trail export, please send a request to support@castoredc.com including the study title for which the export should be created.