Studies overview in eConsent
After logging in, the “Studies” overview will be displayed. The overview will show the list of the studies to which you have been invited to participate.
Studies overview consists of the following elements:
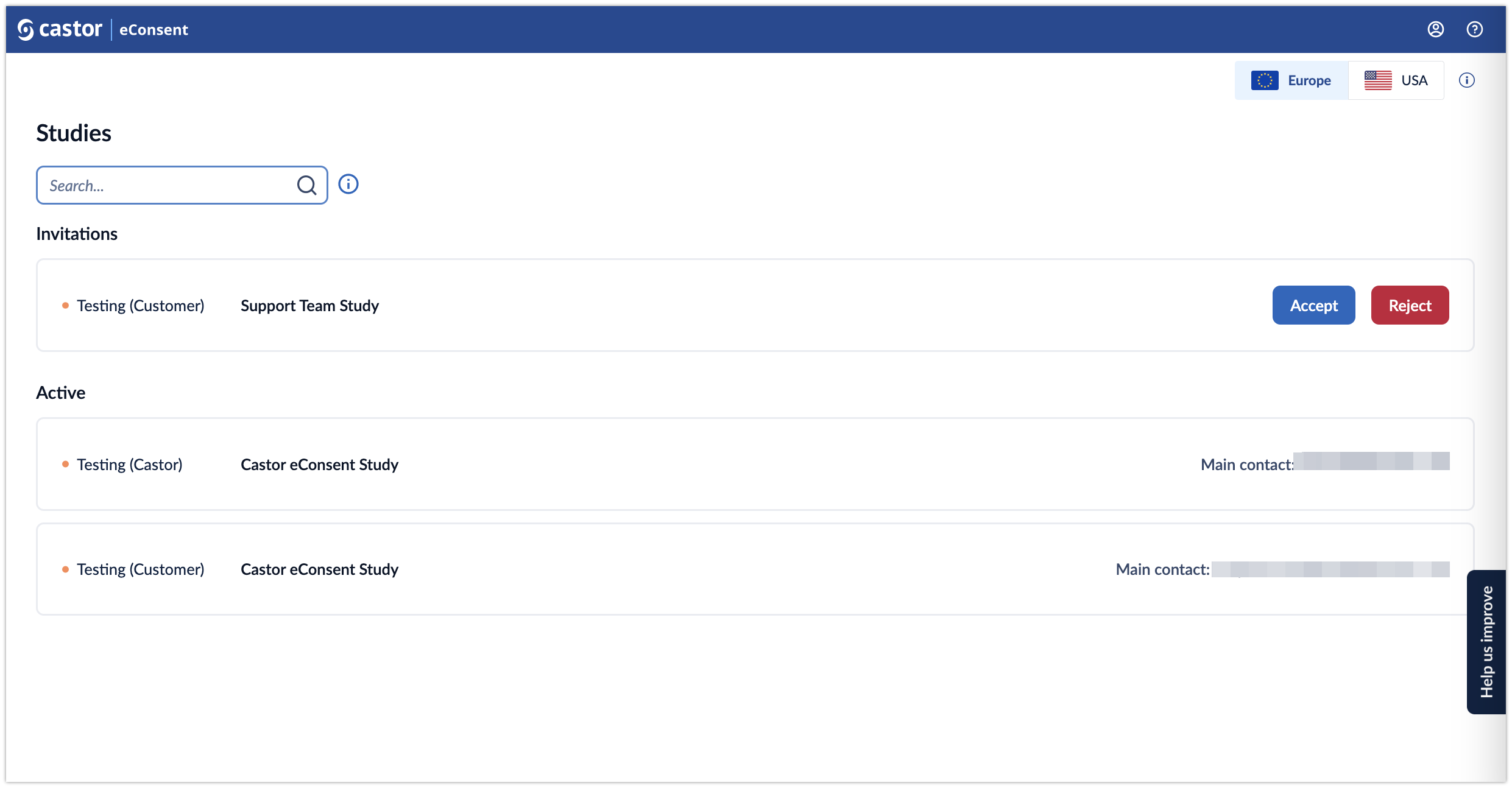
- Servers: Here the data storage locations are displayed. There are two server locations available: Europe and the United States. Study data is only stored on one of our servers and only one server location can be accessed at a time. If you don’t see any studies please make sure to check a different server location.
- Search bar: it is possible to search by organization name, study contact email address or study name
- Invitations: If you are invited to a study, but you have not accepted the invite yet, the study will be listed in the top panel. It is possible to either accept or reject an invitation to a study. Once you have accepted the invitation, you will be able to open a study.
-
Studies: once you have accepted an invitation to a study, it will appear in the ‘Active’ list. The list displays all studies you have access to. For each study, the following information is shown:
- Status: Testing (Castor), Testing (Customer), Live. Read more about what each status means in the article: Study status in eConsent
- Study title: name of the study
- Main contact: main contact of the study. If the main contact has been invited to the study, but have not accepted the invitation yet, the status will be ‘Main contact invited’
-
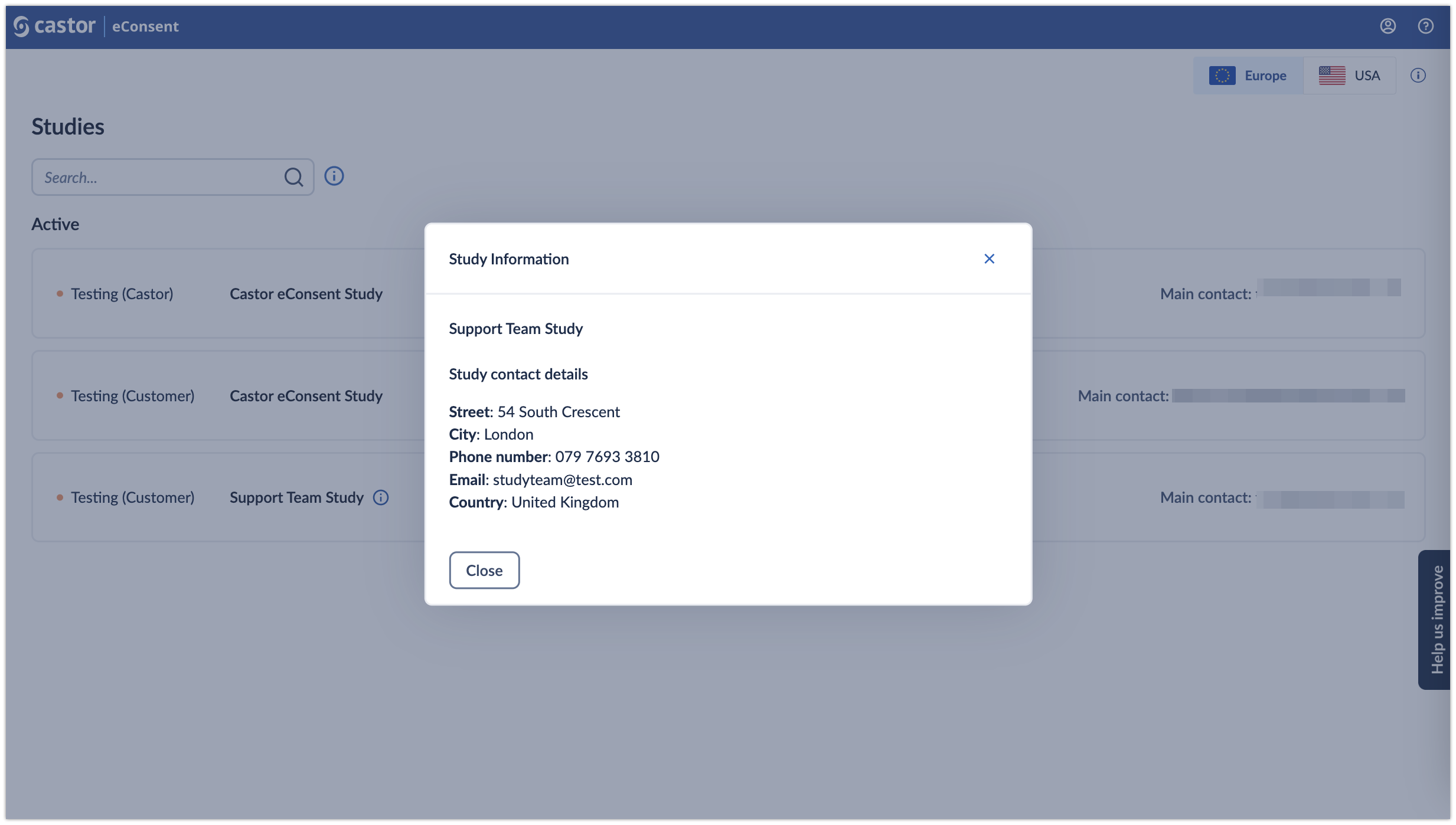
Study information: by clicking on the
 (i) icon, it is possible to view additional study information
(i) icon, it is possible to view additional study information